High-throughput cell optoporation system based on Au nanoparticle layers mediated by resonant irradiation for precise and controllable gene delivery
- PMID: 38321124
- PMCID: PMC10847436
- DOI: 10.1038/s41598-024-53126-9
High-throughput cell optoporation system based on Au nanoparticle layers mediated by resonant irradiation for precise and controllable gene delivery
Abstract
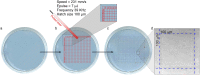
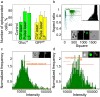
The development of approaches based on genetically modified cells is accompanied by a constant intensive search for new effective and safe delivery systems and the study of existing ones. Recently, we developed a new plasmonic nanoparticle layers-mediated optoporation system that can be proposed for precisely controlled, high-performance laser transfection compatible with broad types of cells and delivered objects of interest. The main goal of the present study is to demonstrate the broad possibilities and advantages of our system for optoporation of several mammalian cells, classified as "easy-to-transfect" cells, namely HeLa and CHO lines, and "hard-to-transfect" cells, namely A431 and RAW 264.7 cells. We show the efficient delivery of various sized cargo molecules: from small molecular dyes propidium iodide (PI) with molecular mass 700 Da, control plasmids (3-10 kb) to fluorophore-labeled dextranes with masses ranging from 10 kDa up to 100 kDa. The performance of optoporation was investigated for two types of laser sources, 800-nm continuous-wave laser, and 1064-nm ns pulsed laser. We provided a comparative study between our system and commercial agent Lipofectamine for transient transfection and stable transfection of HeLa cells with plasmids encoding fluorescent proteins. The quantitative data analysis using flow cytometry, Alamar blue viability assay, and direct fluorescence microscopy revealed higher optoporation efficacy for hard-to-transfect A431 cells and Raw 264.7 cells than lipofection efficacy. Finally, we demonstrated the optoporation performance at the single-cell level by successful delivering PI to the individual CHO cells with revealed high viability for at least 72 h post-irradiation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

