Rebaudioside D decreases adiposity and hepatic lipid accumulation in a mouse model of obesity
- PMID: 38321177
- PMCID: PMC10847429
- DOI: 10.1038/s41598-024-53587-y
Rebaudioside D decreases adiposity and hepatic lipid accumulation in a mouse model of obesity
Abstract
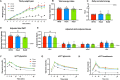
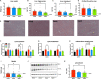
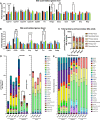
Overconsumption of added sugars has been pointed out as a major culprit in the increasing rates of obesity worldwide, contributing to the rising popularity of non-caloric sweeteners. In order to satisfy the growing demand, industrial efforts have been made to purify the sweet-tasting molecules found in the natural sweetener stevia, which are characterized by a sweet taste free of unpleasant aftertaste. Although the use of artificial sweeteners has raised many concerns regarding metabolic health, the impact of purified stevia components on the latter remains poorly studied. The objective of this project was to evaluate the impact of two purified sweet-tasting components of stevia, rebaudioside A and D (RebA and RebD), on the development of obesity, insulin resistance, hepatic health, bile acid profile, and gut microbiota in a mouse model of diet-induced obesity. Male C57BL/6 J mice were fed an obesogenic high-fat/high-sucrose (HFHS) diet and orally treated with 50 mg/kg of RebA, RebD or vehicle (water) for 12 weeks. An additional group of chow-fed mice treated with the vehicle was included as a healthy reference. At weeks 10 and 12, insulin and oral glucose tolerance tests were performed. Liver lipids content was analyzed. Whole-genome shotgun sequencing was performed to profile the gut microbiota. Bile acids were measured in the feces, plasma, and liver. Liver lipid content and gene expression were analyzed. As compared to the HFHS-vehicle treatment group, mice administered RebD showed a reduced weight gain, as evidenced by decreased visceral adipose tissue weight. Liver triglycerides and cholesterol from RebD-treated mice were lower and lipid peroxidation was decreased. Interestingly, administration of RebD was associated with a significant enrichment of Faecalibaculum rodentium in the gut microbiota and an increased secondary bile acid metabolism. Moreover, RebD decreased the level of lipopolysaccharide-binding protein (LBP). Neither RebA nor RebD treatments were found to impact glucose homeostasis. The daily consumption of two stevia components has no detrimental effects on metabolic health. In contrast, RebD treatment was found to reduce adiposity, alleviate hepatic steatosis and lipid peroxidation, and decrease LBP, a marker of metabolic endotoxemia in a mouse model of diet-induced obesity.
© 2024. The Author(s).
Conflict of interest statement
PDC is inventor on patent applications dealing with the use of specific bacteria and components in the treatment of different diseases. PDC was cofounder of The Akkermansia Company SA and Enterosys SA.
Figures
References
-
- World Health Organization. Obesity and Overweight. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. 2020.
-
- Fagherazzi G, et al. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l'Education Nationale-European prospective investigation into cancer and nutrition cohort. Am. J. Clin. Nutr. 2013;97(3):517–523. doi: 10.3945/ajcn.112.050997. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

