Severe disease during both primary and secondary dengue virus infections in pediatric populations
- PMID: 38321219
- PMCID: PMC7617637
- DOI: 10.1038/s41591-024-02798-x
Severe disease during both primary and secondary dengue virus infections in pediatric populations
Abstract
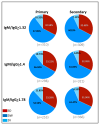
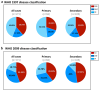
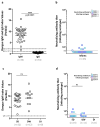
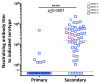
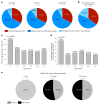
Dengue is a global epidemic causing over 100 million cases annually. The clinical symptoms range from mild fever to severe hemorrhage and shock, including some fatalities. The current paradigm is that these severe dengue cases occur mostly during secondary infections due to antibody-dependent enhancement after infection with a different dengue virus serotype. India has the highest dengue burden worldwide, but little is known about disease severity and its association with primary and secondary dengue infections. To address this issue, we examined 619 children with febrile dengue-confirmed infection from three hospitals in different regions of India. We classified primary and secondary infections based on IgM:IgG ratios using a dengue-specific enzyme-linked immunosorbent assay according to the World Health Organization guidelines. We found that primary dengue infections accounted for more than half of total clinical cases (344 of 619), severe dengue cases (112 of 202) and fatalities (5 of 7). Consistent with the classification based on binding antibody data, dengue neutralizing antibody titers were also significantly lower in primary infections compared to secondary infections (P ≤ 0.0001). Our findings question the currently widely held belief that severe dengue is associated predominantly with secondary infections and emphasizes the importance of developing vaccines or treatments to protect dengue-naive populations.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. WHO; 2009. - PubMed
MeSH terms
Substances
Grants and funding
- IA/E/18/1/504307/WTDBT_/DBT-Wellcome Trust India Alliance/India
- IA/S/14/1/501291/WTDBT_/DBT-Wellcome Trust India Alliance/India
- WT_/Wellcome Trust/United Kingdom
- AI090023/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- 1UO1A/115654/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Medical

