Severe pediatric COVID-19: a review from the clinical and immunopathophysiological perspectives
- PMID: 38321331
- PMCID: PMC11052880
- DOI: 10.1007/s12519-023-00790-y
Severe pediatric COVID-19: a review from the clinical and immunopathophysiological perspectives
Abstract
Background: Coronavirus disease 2019 (COVID-19) tends to have mild presentations in children. However, severe and critical cases do arise in the pediatric population with debilitating systemic impacts and can be fatal at times, meriting further attention from clinicians. Meanwhile, the intricate interactions between the pathogen virulence factors and host defense mechanisms are believed to play indispensable roles in severe COVID-19 pathophysiology but remain incompletely understood.
Data sources: A comprehensive literature review was conducted for pertinent publications by reviewers independently using the PubMed, Embase, and Wanfang databases. Searched keywords included "COVID-19 in children", "severe pediatric COVID-19", and "critical illness in children with COVID-19".
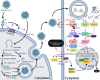
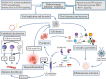
Results: Risks of developing severe COVID-19 in children escalate with increasing numbers of co-morbidities and an unvaccinated status. Acute respiratory distress stress and necrotizing pneumonia are prominent pulmonary manifestations, while various forms of cardiovascular and neurological involvement may also be seen. Multiple immunological processes are implicated in the host response to COVID-19 including the type I interferon and inflammasome pathways, whose dysregulation in severe and critical diseases translates into adverse clinical manifestations. Multisystem inflammatory syndrome in children (MIS-C), a potentially life-threatening immune-mediated condition chronologically associated with COVID-19 exposure, denotes another scientific and clinical conundrum that exemplifies the complexity of pediatric immunity. Despite the considerable dissimilarities between the pediatric and adult immune systems, clinical trials dedicated to children are lacking and current management recommendations are largely adapted from adult guidelines.
Conclusions: Severe pediatric COVID-19 can affect multiple organ systems. The dysregulated immune pathways in severe COVID-19 shape the disease course, epitomize the vast functional diversity of the pediatric immune system and highlight the immunophenotypical differences between children and adults. Consequently, further research may be warranted to adequately address them in pediatric-specific clinical practice guidelines.
Keywords: Immunopathophysiology; MIS-C; Pediatric critical care; Severe pediatric COVID-19.
© 2024. The Author(s).
Conflict of interest statement
Author SQ is a member of the Editorial Board for World Journal of Pediatrics. The paper was handled by the other Editor and has undergone rigorous peer review process. Author SQ was not involved in the journal's review of, or decisions related to, this manuscript. No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors declared no conflicts of interest.
Figures

Similar articles
-
Current Understanding of Multisystem Inflammatory Syndrome (MIS-C) Following COVID-19 and Its Distinction from Kawasaki Disease.Curr Rheumatol Rep. 2021 Jul 3;23(8):58. doi: 10.1007/s11926-021-01028-4. Curr Rheumatol Rep. 2021. PMID: 34216296 Free PMC article. Review.
-
Severe COVID-19 in pediatric age: an update on the role of the anti-rheumatic agents.Pediatr Rheumatol Online J. 2021 May 4;19(1):68. doi: 10.1186/s12969-021-00559-5. Pediatr Rheumatol Online J. 2021. PMID: 33947420 Free PMC article. Review.
-
Age-related differences in the immune response could contribute to determine the spectrum of severity of COVID-19.Immun Inflamm Dis. 2021 Jun;9(2):331-339. doi: 10.1002/iid3.404. Epub 2021 Feb 10. Immun Inflamm Dis. 2021. PMID: 33566457 Free PMC article. Review.
-
Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Antibody Responses in Children With Multisystem Inflammatory Syndrome in Children (MIS-C) and Mild and Severe Coronavirus Disease 2019 (COVID-19).J Pediatric Infect Dis Soc. 2021 May 28;10(5):669-673. doi: 10.1093/jpids/piaa161. J Pediatric Infect Dis Soc. 2021. PMID: 33263756 Free PMC article.
-
Immunology of SARS-CoV-2 infection in children.Nat Immunol. 2022 Feb;23(2):177-185. doi: 10.1038/s41590-021-01123-9. Epub 2022 Feb 1. Nat Immunol. 2022. PMID: 35105983 Free PMC article. Review.
Cited by
-
A retrospective evaluation of SwePEWS use in paediatric patients with COVID-19 and RSV infection.Acta Paediatr. 2025 Feb;114(2):410-416. doi: 10.1111/apa.17450. Epub 2024 Oct 7. Acta Paediatr. 2025. PMID: 39373306 Free PMC article.
-
Histopathological examination of lung from infant with lethal COVID-19 with special attention on pneumocytes type II and the immune infiltrate: a case study.Ital J Pediatr. 2025 Jun 7;51(1):174. doi: 10.1186/s13052-025-01984-y. Ital J Pediatr. 2025. PMID: 40483512 Free PMC article.
-
Diagnostic Markers of Severe COVID-19 and Community-Acquired Pneumonia in Children From Southern India.Microbiol Immunol. 2025 Mar;69(3):174-181. doi: 10.1111/1348-0421.13198. Epub 2025 Jan 15. Microbiol Immunol. 2025. PMID: 39812381 Free PMC article.
-
Paxlovid for the treatment of severe or critical COVID-19 in children.BMC Pediatr. 2025 Jul 2;25(1):493. doi: 10.1186/s12887-025-05807-1. BMC Pediatr. 2025. PMID: 40597122 Free PMC article.
-
Association Between Single-Nucleotide Polymorphisms in Toll-like Receptor 3 (tlr3), tlr7, tlr8 and tirap Genes with Severe Symptoms in Children Presenting COVID-19.Viruses. 2024 Dec 30;17(1):35. doi: 10.3390/v17010035. Viruses. 2024. PMID: 39861824 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

