qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in the emergency department: a randomized controlled trial
- PMID: 38321472
- PMCID: PMC10848347
- DOI: 10.1186/s13054-024-04825-2
qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in the emergency department: a randomized controlled trial
Abstract
Background: Sepsis guidelines suggest immediate start of resuscitation for patients with quick Sequential Organ Failure Assessment (qSOFA) 2 or 3. However, the interpretation of qSOFA 1 remains controversial. We investigated whether measurements of soluble urokinase plasminogen activator receptor (suPAR) may improve risk detection when qSOFA is 1.
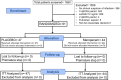
Methods: The study had two parts. At the first part, the combination of suPAR with qSOFA was analyzed in a prospective cohort for early risk detection. At the second part, the double-blind, randomized controlled trial (RCT) SUPERIOR evaluated the efficacy of the suPAR-guided medical intervention. SUPERIOR took place between November 2018 and December 2020. Multivariate stepwise Cox regression was used for the prospective cohort, while univariate and multivariate logistic regression was used for the RCT. Consecutive admissions at the emergency department (ED) with suspected infection, qSOFA 1 and suPAR ≥ 12 ng/mL were allocated to single infusion of placebo or meropenem. The primary endpoint was early deterioration, defined as at least one-point increase of admission Sequential Organ Failure Assessment (SOFA) score the first 24 h.
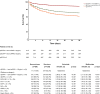
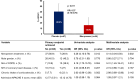
Results: Most of the mortality risk was for patients with qSOFA 2 and 3. Taking the hazard ratio (HR) for death of patients with qSOFA = 1 and suPAR < 12 ng/mL as reference, the HR of qSOFA = 1 and suPAR ≥ 12 ng/mL for 28-day mortality was 2.98 (95% CI 2.11-3.96). The prospective RCT was prematurely ended due to pandemia-related ED re-allocations, with 91 patients enrolled: 47 in the placebo and 44 in the meropenem arm. The primary endpoint was met in 40.4% (n = 19) and 15.9% (n = 7), respectively (difference 24.5% [5.9-40.8]; odds ratio 0.14 [0.04-0.50]). One post hoc analysis showed significant median changes of SOFA score after 72 and 96 h equal to 0 and - 1, respectively.
Conclusions: Combining qSOFA 1 with the biomarker suPAR improves its prognostic performance for unfavorable outcome and can help decision for earlier treatment. Trial registration EU Clinical Trials Register (EudraCT, 2018-001008-13) and Clinical-Trials.gov (NCT03717350). Registered 24 October 2018.
Keywords: Meropenem; Risk; Sepsis; suPAR.
© 2024. The Author(s).
Conflict of interest statement
EJG-Β has received honoraria from Abbott Products Operations, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH and Sobi; independent educational grants from Abbott Products Operations, AbbVie, bioMérieux Inc, InCyte, Johnson & Johnson, MSD, UCD and Sobi; and funding from the Horizon 2020 Marie Skłodowska-Curie International Training Network “the European Sepsis Academy” (granted to the National and Kapodistrian University of Athens), the Horizon 2020 European Grants ImmunoSep and RISC in COVID and the Horizon Health grant EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis). JE-O is a co-founder, shareholder and CSO of ViroGates, Denmark, and named inventor on patents on suPAR owned by Copenhagen University Hospital Hvidovre, Denmark. The other authors do not disclose any conflict of interest.
Figures
Comment in
-
qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in emergency department: a bit sweet and a bit sour randomized controlled trial.Crit Care. 2024 May 13;28(1):161. doi: 10.1186/s13054-024-04944-w. Crit Care. 2024. PMID: 38741141 Free PMC article. No abstract available.
-
Early sepsis recognition: how difficult can this be?Crit Care. 2024 Jun 5;28(1):190. doi: 10.1186/s13054-024-04975-3. Crit Care. 2024. PMID: 38840224 Free PMC article. No abstract available.
References
-
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. CritCareMed. 1985;13(10):818–829. - PubMed
-
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. IntensiveCareMed. 1996;22(7):707–710. - PubMed
-
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study [published correction appears in JAMA 1994 May 4;271(17):1321] JAMA. 1993;270(24):2957–2963. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

