Oral administration of a recombinant modified RBD antigen of SARS-CoV-2 as a possible immunostimulant for the care of COVID-19
- PMID: 38321489
- PMCID: PMC10848483
- DOI: 10.1186/s12934-024-02320-5
Oral administration of a recombinant modified RBD antigen of SARS-CoV-2 as a possible immunostimulant for the care of COVID-19
Abstract
Background: Developing effective vaccines against SARS-CoV-2 that consider manufacturing limitations, equitable access, and acceptance is necessary for developing platforms to produce antigens that can be efficiently presented for generating neutralizing antibodies and as a model for new vaccines.
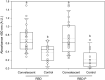
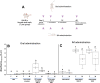
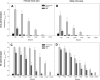
Results: This work presents the development of an applicable technology through the oral administration of the SARS-CoV-2 RBD antigen fused with a peptide to improve its antigenic presentation. We focused on the development and production of the recombinant receptor binding domain (RBD) produced in E. coli modified with the addition of amino acids extension designed to improve antigen presentation. The production was carried out in shake flask and bioreactor cultures, obtaining around 200 mg/L of the antigen. The peptide-fused RBD and peptide-free RBD proteins were characterized and compared using SDS-PAGE gel, high-performance chromatography, and circular dichroism. The peptide-fused RBD was formulated in an oil-in-water emulsion for oral mice immunization. The peptide-fused RBD, compared to RBD, induced robust IgG production in mice, capable of recognizing the recombinant RBD in Enzyme-linked immunosorbent assays. In addition, the peptide-fused RBD generated neutralizing antibodies in the sera of the dosed mice. The formulation showed no reactive episodes and no changes in temperature or vomiting.
Conclusions: Our study demonstrated the effectiveness of the designed peptide added to the RBD to improve antigen immunostimulation by oral administration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The Influence of Adjuvant Type on the Immunogenicity of RBD/N Cocktail Antigens as a Vaccine Candidate against SARS-CoV-2 Virus.Microbiol Spectr. 2023 Jun 15;11(3):e0256422. doi: 10.1128/spectrum.02564-22. Epub 2023 May 18. Microbiol Spectr. 2023. PMID: 37199661 Free PMC article.
-
An Escherichia coli Expressed Multi-Disulfide Bonded SARS-CoV-2 RBD Shows Native-like Biophysical Properties and Elicits Neutralizing Antisera in a Mouse Model.Int J Mol Sci. 2022 Dec 12;23(24):15744. doi: 10.3390/ijms232415744. Int J Mol Sci. 2022. PMID: 36555383 Free PMC article.
-
Recombinant COVID-19 vaccine based on recombinant RBD/Nucleoprotein and saponin adjuvant induces long-lasting neutralizing antibodies and cellular immunity.Front Immunol. 2022 Sep 8;13:974364. doi: 10.3389/fimmu.2022.974364. eCollection 2022. Front Immunol. 2022. PMID: 36159845 Free PMC article.
-
Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies.Biomed Pharmacother. 2020 Oct;130:110559. doi: 10.1016/j.biopha.2020.110559. Epub 2020 Aug 1. Biomed Pharmacother. 2020. PMID: 32768882 Free PMC article. Review.
-
Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19.Hum Vaccin Immunother. 2020 Jun 2;16(6):1239-1242. doi: 10.1080/21645515.2020.1740560. Epub 2020 Apr 16. Hum Vaccin Immunother. 2020. PMID: 32298218 Free PMC article. Review.
References
-
- JHU G. Coronavirus COVID-19 global cases by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU); 2020 https://coronavirus.jhu.edu/map.html. Accessed 29 Sept 2023.
-
- Valdez-Cruz NA, García-Hernández E, Espitia C, Cobos-Marín L, Altamirano C, Bando-Campos CG, Cofas-Vargas Luis F, Coronado-Aceves EW, González-Hernández RA, Hernández-Peralta P, Juárez-López D, Ortega-Portilla PA, Restrepo-Pineda S, Zelada-Cordero P, Trujillo-Roldán MA. Integrative overview of antibodies against SARS-CoV-2 and their possible applications in COVID-19 prophylaxis and treatment. Microb Cell Fact. 2021;20(1):1–32. doi: 10.1186/s12934-021-01576-5. - DOI - PMC - PubMed
-
- Gottlieb M, Wang RC, Yu H, Spatz ES, Montoy JCC, Rodriguez RM, Chang AM, Elmore JG, Hannikainen PA, Hill M, Huebinger RM, Idris AH, Lin Z, Koo K, McDonal’ S, O’Laughlin KN, Plumb ID, Santangelo M, Saydah S, Willis M, Wisk LE, Venkatesh A, Stephens KA, Weinstein RA, Innovative Support for Patients with SARS-CoV-2 Infections Registry (INSPIRE) Group Severe fatigue and persistent symptoms at 3 months following severe acute respiratory syndrome coronavirus 2 infections during the pre-delta, delta, and omicron time periods: a multicenter prospective cohort study. Clin Infect Dis. 2023;76(11):1930–1941. doi: 10.1093/cid/ciad045. - DOI - PMC - PubMed
-
- Lau JJ, Cheng SMS, Leung K, Lee CK, Hachim A, Tsang LCH, Yam KWH, Chaothai S, Kwan KKH, Chai ZYH, Lo THK, Mori M, Wu C, Valkenburg SA, Amarasinghe GK, Lau EHY, Hui DSC, Leung GM, Peiris M, Wu JT. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat Med. 2023;29(2):348–357. doi: 10.1038/s41591-023-02219-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

