Identification of specific markers for human pluripotent stem cell-derived small extracellular vesicles
- PMID: 38321535
- PMCID: PMC10847391
- DOI: 10.1002/jev2.12409
Identification of specific markers for human pluripotent stem cell-derived small extracellular vesicles
Erratum in
-
Correction to article pagination in the Journal of Extracellular Vesicles.J Extracell Vesicles. 2024 May;13(5):e12443. doi: 10.1002/jev2.12443. J Extracell Vesicles. 2024. PMID: 38695388 Free PMC article. No abstract available.
Abstract
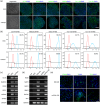
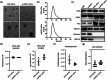
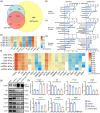
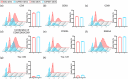
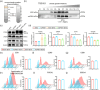
Pluripotent stem cell-derived small extracellular vesicles (PSC-sEVs) have demonstrated great clinical translational potential in multiple aging-related degenerative diseases. Characterizing the PSC-sEVs is crucial for their clinical applications. However, the specific marker pattern of PSC-sEVs remains unknown. Here, the sEVs derived from two typical types of PSCs including induced pluripotent stem cells (iPSC-sEVs) and embryonic stem cells (ESC-sEVs) were analysed using proteomic analysis by liquid chromatography with tandem mass spectrometry (LC-MS/MS), and surface marker phenotyping analysis by nanoparticle flow cytometry (NanoFCM). A group of pluripotency-related proteins were found to be enriched in PSC-sEVs by LC-MS/MS and then validated by Western Blot analysis. To investigate whether these proteins were specifically expressed in PSC-sEVs, sEVs derived from seven types of non-PSCs (non-PSC-sEVs) were adopted for analysis. The results showed that PODXL, OCT4, Dnmt3a, and LIN28A were specifically enriched in PSC-sEVs but not in non-PSC-sEVs. Then, commonly used surface antigens for PSC identification (SSEA4, Tra-1-60 and Tra-1-81) and PODXL were gauged at single-particle resolution by NanoFCM for surface marker identification. The results showed that the positive rates of PODXL (>50%) and SSEA4 (>70%) in PSC-sEVs were much higher than those in non-PSC-sEVs (<10%). These results were further verified with samples purified by density gradient ultracentrifugation. Taken together, this study for the first time identified a cohort of specific markers for PSC-sEVs, among which PODXL, OCT4, Dnmt3a and LIN28A can be detected with Western Blot analysis, and PODXL and SSEA4 can be detected with NanoFCM analysis. The application of these specific markers for PSC-sEVs identification may advance the clinical translation of PSCs-sEVs.
Keywords: pluripotent stem cells; single-particle resolution; small extracellular vesicles; specific markers.
© 2024 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
Yang Wang holds research grants from Shanghai EOOXOM Biotechnology Co., Ltd. Other authors declare no conflict of interest.
Figures


References
-
- Adamiak, M. , Cheng, G. , Bobis‐Wozowicz, S. , Zhao, L. , Kedracka‐Krok, S. , Samanta, A. , Karnas, E. , Xuan, Y. T. , Skupien‐Rabian, B. , Chen, X. , Jankowska, U. , Girgis, M. , Sekula, M. , Davani, A. , Lasota, S. , Vincent, R. J. , Sarna, M. , Newell, K. L. , Wang, O. L. , … Zuba‐Surma, E. K. (2018). Induced pluripotent stem cell (iPSC)‐derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circulation Research, 122(2), 296–309. - PMC - PubMed
-
- Branscome, H. , Paul, S. , Khatkar, P. , Kim, Y. , Barclay, R. A. , Pinto, D. O. , Yin, D. , Zhou, W. , Liotta, L. A. , El‐Hage, N. , & Kashanchi, F. (2020). Stem cell extracellular vesicles and their potential to contribute to the repair of damaged CNS cells. Journal of Neuroimmune Pharmacology, 15(3), 520–537. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

