Generalized myasthenia gravis with acetylcholine receptor antibodies: A guidance for treatment
- PMID: 38321574
- PMCID: PMC11236053
- DOI: 10.1111/ene.16229
Generalized myasthenia gravis with acetylcholine receptor antibodies: A guidance for treatment
Abstract
Background: Generalized myasthenia gravis (MG) with antibodies against the acetylcholine receptor is a chronic disease causing muscle weakness. Access to novel treatments warrants authoritative treatment recommendations. The Nordic countries have similar, comprehensive health systems, mandatory health registers, and extensive MG research.
Methods: MG experts and patient representatives from the five Nordic countries formed a working group to prepare treatment guidance for MG based on a systematic literature search and consensus meetings.
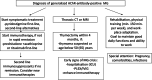
Results: Pyridostigmine represents the first-line symptomatic treatment, while ambenonium and beta adrenergic agonists are second-line options. Early thymectomy should be undertaken if a thymoma, and in non-thymoma patients up to the age of 50-65 years if not obtaining remission on symptomatic treatment. Most patients need immunosuppressive drug treatment. Combining corticosteroids at the lowest possible dose with azathioprine is recommended, rituximab being an alternative first-line option. Mycophenolate, methotrexate, and tacrolimus represent second-line immunosuppression. Plasma exchange and intravenous immunoglobulin are used for myasthenic crises and acute exacerbations. Novel complement inhibitors and FcRn blockers are effective and fast-acting treatments with promising safety profiles. Their use depends on local availability, refunding policies, and cost-benefit analyses. Adapted physical training is recommended. Planning of pregnancies with optimal treatment, information, and awareness of neonatal MG is necessary. Social support and adaptation of work and daily life activities are recommended.
Conclusions: Successful treatment of MG rests on timely combination of different interventions. Due to spontaneous disease fluctuations, comorbidities, and changes in life conditions, regular long-term specialized follow-up is needed. Most patients do reasonably well but there is room for further improvement. Novel treatments are promising, though subject to restricted access due to costs.
Keywords: acetylcholine receptor antibodies; immunosuppression; myasthenia gravis; thymus; treatment.
© 2024 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
N.E.G. has received financial support from UCB, Argenx, Janssen, Merck, Roche, Alexion, Immunovant, Octapharma, Huma, Denka, Grifols, and Dianthus. H.A. has received financial support from UCB, Argenx, Roche, Horizon Therapeutics, Lundbeck, Novo, Alexion, Sanofi‐Genzyme, NMD Pharma, and Octapharma. M.B. has received consultant fees from Argenx and as an investigator for UCB. S.L. has received lecture fees from Argenx, Biogen, Janssen, Merck, Novartis, Roche; congress expenses from Merck, Novartis; advisory fees from Argenx, Novartis, Roche, Sanofi, and UCB; and serves as an investigator for the clinical study Clarion (Merck) and subinvestigator for the clinical study Fenhance (Roche). F.P. has received research grants from Janssen, Merck KGaA, and UCB, and fees for serving on data monitoring committees in clinical trials with Chugai, Lundbeck, and Roche, and preparation of expert witness statement for Novartis. T.H.P. has received a research grant from Octapharma and speaker's honoraria from Argenx and Alexion. A.R.P. has received consultancy fees from Argenx, UCB, Dianthus, and Toleranzia. L.K.A., M.O.L., S.M., and L.S. report no conflicts of interest. J.V. has been a paid consultant for UCB, Horizon Therapeutics, Argenx, Janssen, Roche, Alexion, Immunovant, and Dianthus, and is the Principal Investigator on clinical trials sponsored by UCB, Argenx, Janssen, Roche, Alexion, Horizon Therapeutics, and Regeneron. The other authors have no conflicts of interest.
Figures
References
-
- Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570‐2581. - PubMed
-
- Verschuuren JJ, Palace J, Murai H, Tannemaat MR, Kaminski HJ, Bril V. Advances and ongoing research in the treatment of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21(2):189‐202. - PubMed
-
- Kerty E, Elsais A, Argov Z, Evoli A, Gilhus NE. EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur J Neurol. 2014;21(5):687‐693. - PubMed
-
- Evoli A, Alboini PE, Damato V, et al. Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci. 2018;1412(1):82‐89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

