Amelioration effect of 18β-Glycyrrhetinic acid on methylation inhibitors in hepatocarcinogenesis -induced by diethylnitrosamine
- PMID: 38322013
- PMCID: PMC10844948
- DOI: 10.3389/fimmu.2023.1206990
Amelioration effect of 18β-Glycyrrhetinic acid on methylation inhibitors in hepatocarcinogenesis -induced by diethylnitrosamine
Abstract
Aim: suppression of methylation inhibitors (epigenetic genes) in hepatocarcinogenesis induced by diethylnitrosamine using glycyrrhetinic acid.
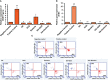
Method: In the current work, we investigated the effect of sole GA combined with different agents such as doxorubicin (DOX) or probiotic bacteria (Lactobacillus rhamanosus) against hepatocarcinogenesis induced by diethylnitrosamine to improve efficiency. The genomic DNA was isolated from rats' liver tissues to evaluate either methylation-sensitive or methylation-dependent resection enzymes. The methylation activity of the targeting genes DLC-1, TET-1, NF-kB, and STAT-3 was examined using specific primers and cleaved DNA products. Furthermore, flow cytometry was used to determine the protein expression profiles of DLC-1 and TET-1 in treated rats' liver tissue.
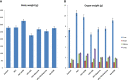
Results: Our results demonstrated the activity of GA to reduce the methylation activity in TET-1 and DLC-1 by 33.6% and 78%, respectively. As compared with the positive control. Furthermore, the association of GA with DOX avoided the methylation activity by 88% and 91% for TET-1 and DLC-1, respectively, as compared with the positive control. Similarly, the combined use of GA with probiotics suppressed the methylation activity in the TET-1 and DLC-1 genes by 75% and 81% for TET-1 and DLC-1, respectively. Also, GA and its combination with bacteria attenuated the adverse effect in hepatocarcinogenesis rats by altering potential methylomic genes such as NF-kb and STAT3 genes by 76% and 83%, respectively.
Conclusion: GA has an ameliorative effect against methylation inhibitors in hepatocellular carcinoma (HCC) by decreasing the methylation activity genes.
Keywords: 18β-Glycyrrhetinic acid (GA); DLC-1 and TET-1; NF-kB; STAT-3 methylation inhibitors; epigenetics; hepatocellular carcinoma (HCC); lactobacillus rhamanosus.
Copyright © 2024 Khalil, Nada, Mahrous, Hassan, Rijo, Ibrahim, Mohamed, AL-Salmi, Mohamed and Elmaksoud.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

