Bystander activation of microglia by Brucella abortus-infected astrocytes induces neuronal death via IL-6 trans-signaling
- PMID: 38322014
- PMCID: PMC10844513
- DOI: 10.3389/fimmu.2023.1343503
Bystander activation of microglia by Brucella abortus-infected astrocytes induces neuronal death via IL-6 trans-signaling
Abstract
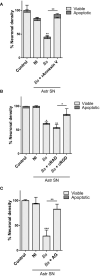
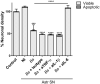
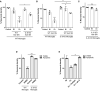
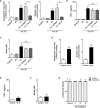
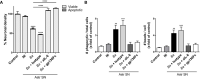
Inflammation plays a key role in the pathogenesis of neurobrucellosis where glial cell interactions are at the root of this pathological condition. In this study, we present evidence indicating that soluble factors secreted by Brucella abortus-infected astrocytes activate microglia to induce neuronal death. Culture supernatants (SN) from B. abortus-infected astrocytes induce the release of pro-inflammatory mediators and the increase of the microglial phagocytic capacity, which are two key features in the execution of live neurons by primary phagocytosis, a recently described mechanism whereby B. abortus-activated microglia kills neurons by phagocytosing them. IL-6 neutralization completely abrogates neuronal loss. IL-6 is solely involved in increasing the phagocytic capacity of activated microglia as induced by SN from B. abortus-infected astrocytes and does not participate in their inflammatory activation. Both autocrine microglia-derived and paracrine astrocyte-secreted IL-6 endow microglial cells with up-regulated phagocytic capacity that allows them to phagocytose neurons. Blocking of IL-6 signaling by soluble gp130 abrogates microglial phagocytosis and concomitant neuronal death, indicating that IL-6 activates microglia via trans-signaling. Altogether, these results demonstrate that soluble factors secreted by B. abortus-infected astrocytes activate microglia to induce, via IL-6 trans-signaling, the death of neurons. IL-6 signaling inhibition may thus be considered a strategy to control inflammation and CNS damage in neurobrucellosis.
Keywords: Brucella abortus; IL-6; astrocytes; microglia; neurobrucellosis; phagocytosis; trans-signaling.
Copyright © 2024 Rodríguez, De Santis Arévalo, Dennis, Rodríguez and Giambartolomei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Brucella abortus-activated microglia induce neuronal death through primary phagocytosis.Glia. 2017 Jul;65(7):1137-1151. doi: 10.1002/glia.23149. Epub 2017 Apr 11. Glia. 2017. PMID: 28398652
-
Brucella abortus induces TNF-α-dependent astroglial MMP-9 secretion through mitogen-activated protein kinases.J Neuroinflammation. 2013 Apr 12;10:47. doi: 10.1186/1742-2094-10-47. J Neuroinflammation. 2013. PMID: 23587438 Free PMC article.
-
Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis.Am J Pathol. 2010 Mar;176(3):1323-38. doi: 10.2353/ajpath.2010.090503. Epub 2010 Jan 21. Am J Pathol. 2010. PMID: 20093491 Free PMC article.
-
The role of NLRP3 and AIM2 in inflammasome activation during Brucella abortus infection.Semin Immunopathol. 2017 Feb;39(2):215-223. doi: 10.1007/s00281-016-0581-1. Epub 2016 Jul 12. Semin Immunopathol. 2017. PMID: 27405866 Free PMC article. Review.
-
Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation.Neuroscientist. 2019 Jun;25(3):227-240. doi: 10.1177/1073858418783959. Epub 2018 Jun 22. Neuroscientist. 2019. PMID: 29931997 Review.
Cited by
-
Neurobrucellosis with negative serological examination: a case report and literature review.Front Med (Lausanne). 2025 Jun 6;12:1583891. doi: 10.3389/fmed.2025.1583891. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40547922 Free PMC article.
-
Microbiota and glioma: a new perspective from association to clinical translation.Gut Microbes. 2024 Jan-Dec;16(1):2394166. doi: 10.1080/19490976.2024.2394166. Epub 2024 Aug 26. Gut Microbes. 2024. PMID: 39185670 Free PMC article. Review.
-
Extracellular vesicles as therapeutic modulators of neuroinflammation in Alzheimer's disease: a focus on signaling mechanisms.J Neuroinflammation. 2025 Apr 25;22(1):120. doi: 10.1186/s12974-025-03443-1. J Neuroinflammation. 2025. PMID: 40281600 Free PMC article. Review.
-
The research trend on neurobrucellosis over the past 30 years (1993-2023): a bibliometric and visualization analysis.Front Neurol. 2024 Sep 23;15:1349530. doi: 10.3389/fneur.2024.1349530. eCollection 2024. Front Neurol. 2024. PMID: 39381075 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

