Immunological insights into COVID-19 in Southern Nigeria
- PMID: 38322252
- PMCID: PMC10844438
- DOI: 10.3389/fimmu.2024.1305586
Immunological insights into COVID-19 in Southern Nigeria
Abstract
Introduction: One of the unexpected outcomes of the COVID-19 pandemic was the relatively low levels of morbidity and mortality in Africa compared to the rest of the world. Nigeria, Africa's most populous nation, accounted for less than 0.01% of the global COVID-19 fatalities. The factors responsible for Nigeria's relatively low loss of life due to COVID-19 are unknown. Also, the correlates of protective immunity to SARS-CoV-2 and the impact of pre-existing immunity on the outcome of the COVID-19 pandemic in Africa are yet to be elucidated. Here, we evaluated the natural and vaccine-induced immune responses from vaccinated, non-vaccinated and convalescent individuals in Southern Nigeria throughout the three waves of the COVID-19 pandemic in Nigeria. We also examined the pre-existing immune responses to SARS-CoV-2 from samples collected prior to the COVID-19 pandemic.
Methods: We used spike RBD and N- IgG antibody ELISA to measure binding antibody responses, SARS-CoV-2 pseudotype assay protocol expressing the spike protein of different variants (D614G, Delta, Beta, Omicron BA1) to measure neutralizing antibody responses and nucleoprotein (N) and spike (S1, S2) direct ex vivo interferon gamma (IFNγ) T cell ELISpot to measure T cell responses.
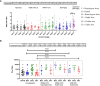
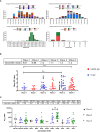
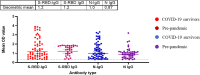
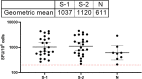
Result: Our study demonstrated a similar magnitude of both binding (N-IgG (74% and 62%), S-RBD IgG (70% and 53%) and neutralizing (D614G (49% and 29%), Delta (56% and 47%), Beta (48% and 24%), Omicron BA1 (41% and 21%)) antibody responses from symptomatic and asymptomatic survivors in Nigeria. A similar magnitude was also seen among vaccinated participants. Interestingly, we revealed the presence of preexisting binding antibodies (N-IgG (60%) and S-RBD IgG (44%)) but no neutralizing antibodies from samples collected prior to the pandemic.
Discussion: These findings revealed that both vaccinated, non-vaccinated and convalescent individuals in Southern Nigeria make similar magnitude of both binding and cross-reactive neutralizing antibody responses. It supported the presence of preexisting binding antibody responses among some Nigerians prior to the COVID-19 pandemic. Lastly, hybrid immunity and heterologous vaccine boosting induced the strongest binding and broadly neutralizing antibody responses compared to vaccine or infection-acquired immunity alone.
Keywords: COVID-19; Nigeria; SARS-CoV-2; immunity; pre-pandemic; preexisting; vaccine.
Copyright © 2024 Ugwu, Alao, John, Akinnawo, Ajayi, Odebode, Bejide, Campbell, Campbell, Adole, B. Olawoye, Akano, Okolie, Eromon, Olaitan, Olagunoye, Adebayo, Adebayo, Babalola, Abioye, Ajayi, Ogah, Ukwaja, Okoro, Oje, Kingsley, Eke, Onyia, Achonduh-Atijegbe, Ewah, Obasi, Igwe, Ayodeji, Chukwuyem, Owhin, Oyejide, Abah, Ingbian, Osoba, Alebiosu, Nadesalingam, Aguinam, Carnell, Krause, Chan, George, Kinsley, Tonks, Temperton, Heeney and Happi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Worldometer . Coronavirus Cases: Deaths: Recovered: Nigeria COVID - Coronavirus Statistics - Worldometer (2023). Available at: https://www.worldometers.info/coronavirus/country/nigeria/.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

