Prognostic value of platelet-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization and tyrosine kinase inhibitors plus immune checkpoints inhibitors
- PMID: 38322419
- PMCID: PMC10844468
- DOI: 10.3389/fonc.2024.1293680
Prognostic value of platelet-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization and tyrosine kinase inhibitors plus immune checkpoints inhibitors
Abstract
Purpose: To investigate the prognostic value of platelet-to-lymphocyte ratio (PLR) in patients with unresectable hepatocellular carcinoma (uHCC) treated with transarterial chemoembolization (TACE) and tailored tyrosine kinase inhibitors (TKIs) plus immune checkpoints inhibitors (ICIs).
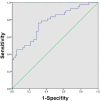
Materials and methods: Ninety-eight patients from May 2018 to January 2022 in our hospital were enrolled in this study. The receiver operating characteristic (ROC) curve analysis was performed and the corresponding Youden index was used to determine the optimal PLR cut-off. Overall survival (OS), progression-free survival (PFS), and adverse events (AEs) of patients were evaluated based on the PLR cut-off. The factors affecting survival were assessed using univariate and multivariate Cox proportional hazards regression analyses.
Results: The PLR cut-off was 98.89. There were 49 patients in the low pretreatment PLR group (PLR ≤ 98.89) and 49 patients in the high PLR group (PLR > 98.89). Patients with low pretreatment PLR had significantly longer median OS (25.7 months vs 16.1 months; P < 0.001) and PFS (14.9 months vs 10.2 months; P < 0.001) than those with high pretreatment PLR. The multivariate analysis revealed that ALT, tumor size, and PLR are risk factors affecting OS. The three independent factors affecting PFS are tumor size, AFP, and PLR. The AEs were tolerable and manageable.
Conclusion: The low pretreatment PLR (PLR ≤ 98.89) was an independent protective factor for the survival outcomes of patients in this study. PLR was helpful for clinicians to predict the prognosis and identify the patients with uHCC who were most likely to benefit from TACE + TKIs + ICIs.
Keywords: hepatocellular carcinoma; immune checkpoint inhibitors; platelet-to-lymphocyte ratio; tailored tyrosine kinase inhibitors; transarterial chemoembolization.
Copyright © 2024 Guo, Wu, Sun, Guo, Si, Zheng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Biomarker, efficacy and safety analysis of transcatheter arterial chemoembolization combined with atezolizumab and bevacizumab for unresectable hepatocellular carcinoma.Cancer Immunol Immunother. 2025 May 19;74(7):209. doi: 10.1007/s00262-025-04058-4. Cancer Immunol Immunother. 2025. PMID: 40387956 Free PMC article.
-
Neutrophil-to-lymphocyte ratio predicts therapy outcomes of transarterial chemoembolization combined with tyrosine kinase inhibitors plus programmed cell death ligand 1 antibody for unresectable hepatocellular carcinoma.Anticancer Drugs. 2023 Jul 1;34(6):775-782. doi: 10.1097/CAD.0000000000001458. Epub 2022 Nov 17. Anticancer Drugs. 2023. PMID: 36730299
-
Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Outcomes in Patients With Unresectable Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization Plus Sorafenib.Front Mol Biosci. 2021 May 28;8:624366. doi: 10.3389/fmolb.2021.624366. eCollection 2021. Front Mol Biosci. 2021. PMID: 34124139 Free PMC article.
-
The Significance of Transarterial Chemo(Embolization) Combined With Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma in the Era of Systemic Therapy: A Systematic Review.Front Immunol. 2022 May 23;13:913464. doi: 10.3389/fimmu.2022.913464. eCollection 2022. Front Immunol. 2022. PMID: 35677059 Free PMC article.
-
Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: A review.Front Oncol. 2022 Sep 28;12:980214. doi: 10.3389/fonc.2022.980214. eCollection 2022. Front Oncol. 2022. PMID: 36249023 Free PMC article. Review.
Cited by
-
Prognostic value of platelet-to-lymphocyte ratio in hepatocellular carcinoma patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis.BMC Gastroenterol. 2025 Jun 6;25(1):437. doi: 10.1186/s12876-025-04028-1. BMC Gastroenterol. 2025. PMID: 40481391 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

