[Mechanobiological Mechanisms Involved in the Regualation of the Blood-Brain Barrier by Fluid Shear Force]
- PMID: 38322523
- PMCID: PMC10839479
- DOI: 10.12182/20240160211
[Mechanobiological Mechanisms Involved in the Regualation of the Blood-Brain Barrier by Fluid Shear Force]
Abstract
Objective: To explore the mechanobiological mechanism of fluid shear force (FSF) on the protection, injury, and destruction of the structure and function of the blood-brain barrier (BBB) under normal physiological conditions, ischemic hypoperfusion, and postoperative hyperperfusion conditions. BBB is mainly composed of brain microvascular endothelial cells. Rat brain microvascular endothelial cells (rBMECs) were used as model cells to conduct the investigation.
Methods: rBMECs were seeded at a density of 1×105 cells/cm2 and incubated for 48 h. FSF was applied to the rBMECs at 0.5, 2, and 20 dyn/cm2, respectively, simulating the stress BBB incurs under low perfusion, normal physiological conditions, and high FSF after bypass grafting when there is cerebral vascular stenosis. In addition, a rBMECs static culture group was set up as the control (no force was applied). Light microscope, scanning electron microscope (SEM), and laser confocal microscope (LSCM) were used to observe the changes in cell morphology and cytoskeleton. Transmission electron microscope (TEM) was used to observe the tight junctions. Immunofluorescence assay was performed to determine changes in the distribution of tight junction-associated proteins claudin-5, occludin, and ZO-1 and adherens junction-associated proteins VE-cadherin and PECAM-1. Western blot was performed to determine the expression levels of tight junction-associated proteins claudin-5, ZO-1, and JAM4, adherens junction-associated protein VE-cadherin, and key proteins in Rho GTPases signaling (Rac1, Cdc42, and RhoA) under FSF at different intensities.
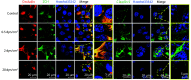
Results: Microscopic observation showed that the cytoskeleton exhibited disorderly arrangement and irregular orientation under static culture and low shear force (0.5 dyn/cm2). Under normal physiological shear force (2 dyn/cm2), the cytoskeleton was rearranged in the orientation of the FSF and an effective tight junction structure was observed between cells. Under high shear force (20 dyn/cm2), the intercellular space was enlarged and no effective tight junction structure was observed. Immunofluorescence results showed that, under low shear force, the gap between the cells decreased, but there was also decreased distribution of tight junction-associated proteins and adherens junction-associated proteins at the intercellular junctions. Under normal physiological conditions, the cells were tightly connected and most of the tight junction-associated proteins were concentrated at the intercellular junctions. Under high shear force, the gap between the cells increased significantly and the tight junction and adherens junction structures were disrupted. According to the Western blot results, under low shear force, the expression levels of claudin-5, ZO-1, and VE-cadherin were significantly up-regulated compared with those of the control group (P<0.05). Under normal physiological shear force, claudin-5, ZO-1, JAM4, and VE-cadherin were highly expressed compared with those of the control group (P<0.05). Under high shear force, the expressions of claudin-5, ZO-1, JAM4, and VE-cadherin were significantly down-regulated compared with those of the normal physiological shear force group (P<0.05). Under normal physiological shear force, intercellular expressions of Rho GTPases proteins (Rac1, Cdc42, and RhoA) were up-regulated and were higher than those of the other experimental groups (P<0.05). The expressions of Rho GTPases under low and high shear forces were down-regulated compared with that of the normal physiological shear force group (P<0.05).
Conclusion: Under normal physiological conditions, FSF helps maintain the integrity of the BBB structure, while low or high shear force can damage or destroy the BBB structure. The regulation of BBB by FSF is closely related to the expression and distribution of tight junction-associated proteins and adherens junction-associated proteins.
目的: 血脑屏障(blood-brain barrier, BBB )主要由脑微血管内皮细胞组成。本研究以大鼠脑微血管内皮细胞(rat brain microvascular endothelial cells, rBMECs)为模式细胞,探索正常生理、缺血低灌注和术后高灌注条件下的流体剪切力(flow shear force, FSF)对 BBB 结构功能的保护、损伤和破坏的力学生物学机制。
方法: 以1×105细胞/cm2的密度接种rBMECs培养48 h,对rBMECs分别施加0.5、2和20 dyn/cm2的FSF,模拟脑血管狭窄的低灌注、正常生理和搭桥术后高剪切力作用下BBB结构的受力情况;同时设置静态培养组rBMECs作为对照(不施加力)。光学显微镜、扫描电子显微镜(SEM)以及激光共聚焦显微镜(LSCM)观察细胞形态和骨架的变化。透射电子显微镜(TEM)观察细胞紧密连接,免疫荧光染色检测紧密连接相关蛋白(claudin-5、occludin、ZO-1)、黏着连接相关蛋白(VE-cadherin、PECAM-1)分布的变化。Western blot检测不同强度FSF下紧密连接相关蛋白(claudin-5、ZO-1、JAM4),黏着连接相关蛋白(VE-cadherin)和Rho GTPases信号关键蛋白(Rac1、Cdc42、RhoA)的表达。
结果: 镜下观察发现:静态培养和低剪切作用(0.5 dyn/cm2)下细胞骨架排列紊乱,取向无规律;正常生理剪切作用(2 dyn/cm2)下,细胞骨架顺应FSF方向重排,胞间观察到有效的紧密连接结构;高剪切作用(20 dyn/cm2)下,细胞间间隙增大,未观察到有效的紧密连接结构。免疫荧光染色发现低剪切作用时,细胞间距减小,但胞间连接处紧密连接相关蛋白和黏着连接相关蛋白分布较少;正常生理条件下细胞连接紧密,大部分紧密连接相关蛋白的分布集中在胞间连接处;高剪切作用时,胞间距离显著增大,连接紧密和黏着连接的结构被破坏。Western blot结果发现:低剪切作用下,claudin-5、ZO-1和VE-cadherin与对照组相比均上调(P<0.05);正常生理剪切作用下,claudin-5、ZO-1、JAM4以及VE-cadherin与对照组相比均呈现高表达(P<0.05);高剪切作用下,claudin-5、ZO-1、JAM4以及VE-cadherin的表达与正常生理剪切作用组比较下调(P<0.05);生理条件下胞内的Rho GTPases(Rac1、Cdc42、RhoA)表达上调,高于其他实验组(P<0.05),而低剪切和高剪切作用下的Rho GTPases表达均较正常生理剪切作用组下调(P<0.05)。
结论: 正常生理条件下的FSF有助于维持BBB结构的完整性,而低剪切或高剪切均会损伤或破坏血脑屏障的结构,FSF对BBB的调控与紧密连接相关蛋白和黏着连接相关蛋白的表达和分布密切相关。
Keywords: Adherens junction-associated proteins; Blood-brain barrier; Fluid shear force; Rat brain microvascular endothelial cells; Tight junction-associated proteins.
© 2024《四川大学学报(医学版)》编辑部 版权所有Copyright ©2024 Editorial Board of Journal of Sichuan University (Medical Sciences).
Conflict of interest statement
利益冲突 所有作者均声明不存在利益冲突
Figures



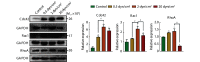
Similar articles
-
Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels.J Cell Physiol. 2011 Nov;226(11):3053-63. doi: 10.1002/jcp.22655. J Cell Physiol. 2011. PMID: 21302304
-
RhoA/ROCK-2 Pathway Inhibition and Tight Junction Protein Upregulation by Catalpol Suppresses Lipopolysaccaride-Induced Disruption of Blood-Brain Barrier Permeability.Molecules. 2018 Sep 17;23(9):2371. doi: 10.3390/molecules23092371. Molecules. 2018. PMID: 30227623 Free PMC article.
-
Partial recovery of the damaged rat blood-brain barrier is mediated by adherens junction complexes, extracellular matrix remodeling and macrophage infiltration following focal astrocyte loss.Neuroscience. 2013 Oct 10;250:773-85. doi: 10.1016/j.neuroscience.2013.06.061. Epub 2013 Jul 9. Neuroscience. 2013. PMID: 23845748 Free PMC article.
-
MicroRNA Regulation of Endothelial Junction Proteins and Clinical Consequence.Mediators Inflamm. 2016;2016:5078627. doi: 10.1155/2016/5078627. Epub 2016 Nov 24. Mediators Inflamm. 2016. PMID: 27999452 Free PMC article. Review.
-
Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets.J Cereb Blood Flow Metab. 2018 Aug;38(8):1255-1275. doi: 10.1177/0271678X18774666. Epub 2018 May 8. J Cereb Blood Flow Metab. 2018. PMID: 29737222 Free PMC article. Review.
References
-
- 王陇德, 刘建民, 杨弋, 等 我国脑卒中防治仍面临巨大挑战——《中国脑卒中防治报告 2018》 概要. 中国循环杂志. 2019;34(2):105–119. doi: 10.3969/j.issn.1000-3614.2019.02.001. - DOI
- WANG L D, LIU J M, YANG Y, et al The prevention and treatment of stroke still face huge challenges --brief report on stroke prevention and treatment in China, 2018. Chin Circulat J. 2019;34(2):105–119. doi: 10.3969/j.issn.1000-3614.2019.02.001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
