Hypermethylated SHOX2 in circulating cell-free DNA post renal cell carcinoma surgery as TNM-independent biomarker for recurrence risk
- PMID: 38322559
- PMCID: PMC10839385
- DOI: 10.62347/QOBT7285
Hypermethylated SHOX2 in circulating cell-free DNA post renal cell carcinoma surgery as TNM-independent biomarker for recurrence risk
Abstract
Introduction: Adjuvant immune checkpoint inhibitor trials in renal cell carcinoma (RCC) call for improved recurrence risk stratification. Due to limitations of circulating tumor DNA (ctDNA) use in RCC, the use of hypermethylated SHOX2 gene (mSHOX2) in circulating cell-free DNA is explored as a surrogate marker for identifying high-risk patients after RCC surgery.
Methods: Liquid biopsies were collected post-surgery from 45 RCC patients (mean duration 4.3 days). Real-time polymerase chain reaction was used to analyze SHOX2 methylation in circulating cell-free DNA. Patients were categorized as mSHOX2 positive or negative by cut-off. Metastasis-free survival (MFS), cancer-specific survival (CSS), and overall survival (OS) were assessed using Cox regression and Log-rank analyses (median follow-up time: 60 months).
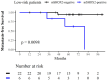
Results: 17 patients were mSHOX2 positive, showing unfavorable OS/CSS (Log-rank P = 0.004 and 0.02) and nearly 6-fold higher recurrence risk (hazard ratio 5.89, 95% CI 1.46-23.8). Multivariable Cox analysis confirmed mSHOX2 as an independent recurrence risk factor, disregarding TNM-based stratification.
Conclusions: mSHOX2 effectively identifies high-risk RCC patients post-surgery, indicating minimal residual disease. This easy to implement biomarker has potential for guiding of adjuvant therapy decisions.
Keywords: Renal cell carcinoma; SHOX2; adjuvant therapy; biomarker; recurrence.
AJTR Copyright © 2024.
Conflict of interest statement
DD has been an employee of Epigenomics AG, Berlin, Germany. This company aims to commercialize DNA methylation biomarker SHOX2. DD is co-inventor and owns patents on methylation biomarkers and related technologies. DD receives inventor’s compensation from Epigenomics AG.
Figures


Similar articles
-
Cell-Free SHOX2 DNA Methylation in Blood as a Molecular Staging Parameter for Risk Stratification in Renal Cell Carcinoma Patients: A Prospective Observational Cohort Study.Clin Chem. 2019 Apr;65(4):559-568. doi: 10.1373/clinchem.2018.297549. Epub 2019 Jan 9. Clin Chem. 2019. PMID: 30626634
-
Circulating Cell-Free SHOX2 DNA Methylation Is a Predictive, Prognostic, and Monitoring Biomarker in Adjuvant and Palliative Anti-PD-1-Treated Melanoma.Clin Chem. 2024 Mar 2;70(3):516-527. doi: 10.1093/clinchem/hvad230. Clin Chem. 2024. PMID: 38300881
-
Early Dynamics of Quantitative SEPT9 and SHOX2 Methylation in Circulating Cell-Free Plasma DNA during Prostate Biopsy for Prostate Cancer Diagnosis.Cancers (Basel). 2022 Sep 7;14(18):4355. doi: 10.3390/cancers14184355. Cancers (Basel). 2022. PMID: 36139516 Free PMC article.
-
The Role of Circulating Tumor DNA in Renal Cell Carcinoma.Curr Treat Options Oncol. 2018 Feb 20;19(2):10. doi: 10.1007/s11864-018-0530-4. Curr Treat Options Oncol. 2018. PMID: 29464405 Review.
-
Nephron-sparing surgery for renal cell carcinoma: clinicopathologic features predictive of patient outcome.Urology. 2003 Oct;62(4):641-6. doi: 10.1016/s0090-4295(03)00489-8. Urology. 2003. PMID: 14550434 Review.
Cited by
-
Japan society of clinical oncology position paper on appropriate clinical use of molecular residual disease (MRD) testing.Int J Clin Oncol. 2025 Apr;30(4):605-654. doi: 10.1007/s10147-024-02683-0. Epub 2025 Feb 7. Int J Clin Oncol. 2025. PMID: 39920551 Free PMC article. Review.
-
Liquid biopsy for Renal Cell Carcinoma: A comprehensive review of techniques, applications, and future prospects.Kidney Cancer. 2024 Feb;8(1):205-225. doi: 10.1177/24684570241303346. Epub 2024 Dec 24. Kidney Cancer. 2024. PMID: 39886007 Free PMC article.
-
Turning the tide: pembrolizumab's triumph in adjuvant RCC therapy.Med Oncol. 2024 Sep 5;41(10):242. doi: 10.1007/s12032-024-02486-3. Med Oncol. 2024. PMID: 39237796 Review.
References
-
- Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Gurney H, Chang YH, Lee JL, Sarwar N, Thiery-Vuillemin A, Gross-Goupil M, Mahave M, Haas NB, Sawrycki P, Burgents JE, Xu L, Imai K, Quinn DI, Choueiri TK, Tomczak P, Choueiri T, Park SH, Venugopal B, Ferguson TR, Hajek J, Lin TP, Symeonides SN, Lee JL, Sawrycki P, Haas NB, Gurney HP, Mahave M, Sarwar N, Thiery-Vuillemin A, Gross-Goupil M, Chevreau C, Burke JM, Doshi G, Melichar B, Topart D, Oudard S, Kopyltsov E, Hammers HJ, Quinn DI, Alva A, Menezes JdJ, Silva AGe, Winquist EW, Hamzaj A, Procopio G, Karaszewska B, Nowakowska-Zajdel EM, Alekseev BY, Gafanov RA, Izmailov A, Semenov A, Afanasyev SG, Lipatov ON, Powles TB, Srinivas S, McDermott D, Kochuparambil ST, Davis ID, Peltola K, Sabbatini R, Chung J, Shkolnik MI, Matveev VB, Gajate Borau P, McCune S, Hutson TE, Dri A, Sales SC, Yeung C, Alcala Castro CM, Bostrom P, Laguerre B, Buttigliero C, de Giorgi U, Fomin EA, Zakharia Y, Hwang C, Singer EA, Yorio JT, Waterhouse D, Kowalyszyn RD, Alfie MS, Yanez Ruiz E, Buchler T, Kankaanranta K, Ferretti G, Kimura G, Nishimura K, Masumori N, Tamada S, Kato H, Kitamura H, Danielewicz I, Wojcik-Tomaszewska J, Sala Gonzalez N, Chiu KY, Atkins MB, Heath E, Rojas-Uribe GA, Gonzalez Fernandez ME, Feyerabend S, Pignata S, Numakura K, Cybulska Stopa B, Zukov R, Climent Duran MA, Maroto Rey PJ, Montesa Pino A, Chang CH, Vengalil S, Waddell TS, Cobb PW, Hauke R, Anderson DM, Sarantopoulos J, Gourdin T, Zhang T, Jayram G, Fein LE, Harris C, Beato PMM, Flores F, Estay A, Rubiano JA, Bedke J, Hauser S, Neisius A, Busch J, Anai S, Tsunemori H, Sawka D, Sikora-Kupis B, Arranz JA, Delgado I, Chen CH, Gunderson E, Tykodi S, Koletsky A, Chen K, Agrawal M, Kaen DL, Sade JP, Tatangelo MD, Parnis F, Barbosa FM, Faucher G, Iqbal N, Marceau D, Paradis JB, Hanna N, Acevedo A, Ibanez C, Villanueva L, Galaz PP, Durango IC, Manneh R, Kral Z, Holeckova P, Hakkarainen H, Ronkainen H, Abadie-Lacourtoisie S, Tartas S, Goebell PJ, Grimm MO, Hoefner T, Wirth M, Panic A, Schultze-Seemann W, Yokomizo A, Mizuno R, Uemura H, Eto M, Tsujihata M, Matsukawa Y, Murakami Y, Kim M, Hamberg P, Marczewska-Skrodzka M, Szczylik C, Humphreys AC, Jiang P, Kumar B, Lu G, Desai A, Karam JA, Keogh G, Fleming M, Zarba JJ, Leiva VE, Mendez GA, Harris SJ, Brown SJ, Antonio Junior JN, Costamilan RdC, Rocha RO, Muniz D, Brust L, Lalani AK, Graham J, Levesque M, Orlandi F, Kotasek R, Deville JL, Borchiellini D, Merseburger A, Rink M, Roos F, McDermott R, Oyama M, Yamamoto Y, Tomita Y, Miura Y, Ioritani N, Westgeest H, Kubiatowski T, Bal W, Girones Sarrio R, Rowe J, Prow DM, Senecal F, Hashemi-Sadraei N, Cole SW, Kendall SD, Richards DA, Schnadig ID, Gupta M. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:1133–1144. - PubMed
-
- Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Ljungberg B, Marconi L, Klatte T, Volpe A, Abu-Ghanem Y, Dabestani S, Fernández-Pello S, Hofmann F, Kuusk T, Tahbaz R, Powles T, Bex A. The 2022 updated european association of urology guidelines on the use of adjuvant immune checkpoint inhibitor therapy for renal cell carcinoma. Eur Urol. 2023;83:10–14. - PubMed
-
- Marchioni M, Amparore D, Marandino L, Bertolo R, Erdem S, Ingels A, Muselaers S, Kara O, Pavan N, Roussel E, Carbonara U, Pecoraro A, Diana P, Pecoraro A, Campi R European Association of Urology Young Academic Urologists Renal Cancer Working Group. Is adjuvant immunotherapy worth for all patients with clear-cell renal cell carcinoma at high risk of recurrence? Eur Urol Open Sci. 2022;46:39–42. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
