Combinatorial immune checkpoint blockade increases myocardial expression of NLRP-3 and secretion of H-FABP, NT-Pro-BNP, interleukin-1β and interleukin-6: biochemical implications in cardio-immuno-oncology
- PMID: 38322766
- PMCID: PMC10844473
- DOI: 10.3389/fcvm.2024.1232269
Combinatorial immune checkpoint blockade increases myocardial expression of NLRP-3 and secretion of H-FABP, NT-Pro-BNP, interleukin-1β and interleukin-6: biochemical implications in cardio-immuno-oncology
Abstract
Background: Immune checkpoint blockade in monotherapy or combinatorial regimens with chemotherapy or radiotherapy have become an integral part of oncology in recent years. Monoclonal antibodies against CTLA-4 or PD-1 or PDL-1 are the most studied ICIs in randomized clinical trials, however, more recently, an anti-LAG3 (Lymphocyte activation gene-3) antibody, Relatlimab, has been approved by FDA in combination with Nivolumab for metastatic melanoma therapy. Moreover, Atezolizumab is actually under study in association with Ipilimumab for therapy of metastatic lung cancer. Myocarditis, vasculitis and endothelitis are rarely observed in these patients on monotherapy, however new combination therapies could expose patients to more adverse cardiovascular events.
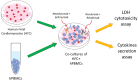
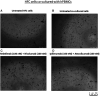
Methods: Human cardiomyocytes co-cultured with human peripheral blood lymphocytes (hPBMCs) were exposed to monotherapy and combinatorial ICIs (PD-L1 and CTLA-4 or PD-1 and LAG-3 blocking agents, at 100 nM) for 48 h. After treatments, cardiac cell lysis and secretion of biomarkers of cardiotoxicity (H-FABP, troponin-T, BNP, NT-Pro-BNP), NLRP3-inflammasome and Interleukin 1 and 6 were determined through colorimetric and enzymatic assays. Mitochondrial functions were studied in cardiomyocyte cell lysates through quantification of intracellular Ca++, ATP content and NADH:ubiquinone oxidoreductase core subunit S1 (Ndufs1) levels. Histone deacetylases type 4 (HDAC-4) protein levels were also determined in cardiomyocyte cell lysates to study potential epigenetic changes induced by immunotherapy regimens.
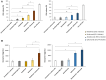
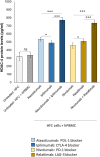
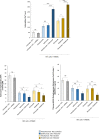
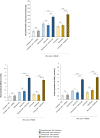
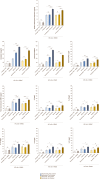
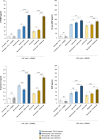
Results: Both combinations of immune checkpoint inhibitors exert more potent cardiotoxic side effects compared to monotherapies against human cardiac cells co-cultured with human lymphocytes. LDH release from cardiac cells was 43% higher in PD-L1/CTLA-4 blocking agents, and 35.7% higher in PD-1/LAG-3 blocking agents compared to monotherapies. HDAC4 and intracellular Ca++ levels were increased, instead ATP content and Ndufs1 were reduced in myocardial cell lysates (p < 0.001 vs. untreated cells). Troponin-T, BNP, NT-Pro-BNP and H-FABP, were also strongly increased in combination therapy compared to monotherapy regimen. NLRP3 expression, IL-6 and IL-1β levels were also increased by PDL-1/CTLA-4 and PD-1/LAG-3 combined blocking agents compared to untreated cells and monotherapies.
Conclusions: Data of the present study, although in vitro, indicate that combinatorial immune checkpoint blockade, induce a pro- inflammatory phenotype, thus indicating that these therapies should be closely monitored by the multidisciplinary team consisting of oncologists, cardiologists and immunologists.
Keywords: cancer; cardiology; cardiotoxicity; immune checkpoint inhibitors; inflammation; myocarditis; oncology.
© 2024 Quagliariello, Passariello, Bisceglia, Paccone, Inno, Maurea, Rapuano Lembo, Manna, Iovine, Canale, Scherillo, Ascierto, Gabrielli, De Lorenzo and Maurea.
Conflict of interest statement
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

