Nature stays natural: two novel chemo-enzymatic one-pot cascades for the synthesis of fragrance and flavor aldehydes
- PMID: 38323304
- PMCID: PMC10840651
- DOI: 10.1039/d3gc04191c
Nature stays natural: two novel chemo-enzymatic one-pot cascades for the synthesis of fragrance and flavor aldehydes
Abstract
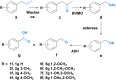
Novel synthetic strategies for the production of high-value chemicals based on the 12 principles of green chemistry are highly desired. Herein, we present a proof of concept for two novel chemo-enzymatic one-pot cascades allowing for the production of valuable fragrance and flavor aldehydes. We utilized renewable phenylpropenes, such as eugenol from cloves or estragole from estragon, as starting materials. For the first strategy, Pd-catalyzed isomerization of the allylic double bond and subsequent enzyme-mediated (aromatic dioxygenase, ADO) alkene cleavage were performed to obtain the desired aldehydes. In the second route, the double bond was oxidized to the corresponding ketone via a copper-free Wacker oxidation protocol followed by enzymatic Baeyer-Villiger oxidation (phenylacetone monooxygenase from Thermobifida fusca), esterase-mediated (esterase from Pseudomonas fluorescens, PfeI) hydrolysis and subsequent oxidation of the primary alcohol (alcohol dehydrogenase from Pseudomonas putida, AlkJ) to the respective aldehyde products. Eight different phenylpropene derivatives were subjected to these reaction sequences, allowing for the synthesis of seven aldehydes in up to 55% yield after 4 reaction steps (86% for each step).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


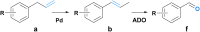

Similar articles
-
Transforming a Historical Chemical Synthetic Route for Vanillin Starting from Renewable Eugenol to a Cell-Free Bi-Enzymatic Cascade.ChemSusChem. 2025 Jun 2;18(11):e202500387. doi: 10.1002/cssc.202500387. Epub 2025 Apr 16. ChemSusChem. 2025. PMID: 40091706 Free PMC article.
-
Biochemical analysis of recombinant AlkJ from Pseudomonas putida reveals a membrane-associated, flavin adenine dinucleotide-dependent dehydrogenase suitable for the biosynthetic production of aliphatic aldehydes.Appl Environ Microbiol. 2014 Apr;80(8):2468-77. doi: 10.1128/AEM.04297-13. Epub 2014 Feb 7. Appl Environ Microbiol. 2014. PMID: 24509930 Free PMC article.
-
Recent advances in artificial enzyme cascades for the production of value-added chemicals.Bioresour Technol. 2021 Mar;323:124551. doi: 10.1016/j.biortech.2020.124551. Epub 2020 Dec 16. Bioresour Technol. 2021. PMID: 33360113 Review.
-
Programing a cyanide-free transformation of aldehydes to nitriles and one-pot synthesis of amides through tandem chemo-enzymatic cascades.RSC Adv. 2022 Jun 16;12(28):17873-17881. doi: 10.1039/d2ra03256b. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35765330 Free PMC article.
-
High-Yield Synthesis of Enantiopure 1,2-Amino Alcohols from l-Phenylalanine via Linear and Divergent Enzymatic Cascades.Org Process Res Dev. 2022 Jul 15;26(7):2085-2095. doi: 10.1021/acs.oprd.1c00490. Epub 2022 Mar 28. Org Process Res Dev. 2022. PMID: 35873603 Free PMC article. Review.
Cited by
-
Transforming a Historical Chemical Synthetic Route for Vanillin Starting from Renewable Eugenol to a Cell-Free Bi-Enzymatic Cascade.ChemSusChem. 2025 Jun 2;18(11):e202500387. doi: 10.1002/cssc.202500387. Epub 2025 Apr 16. ChemSusChem. 2025. PMID: 40091706 Free PMC article.
-
Structural and Functional Characteristics of Potent Dioxygenase from Moesziomyces aphidis.JACS Au. 2025 Jun 12;5(7):3014-3020. doi: 10.1021/jacsau.5c00456. eCollection 2025 Jul 28. JACS Au. 2025. PMID: 40747015 Free PMC article.
References
-
- IEA, The Future of Petrochemicals, IEA, Paris, 2018
-
- Anastas P. T. and Warner J. C., Green Chemistry: Theory and Practice, Oxford University Press, 1998
-
- Bruggink A. Schoevaart R. Kieboom T. Org. Process Res. Dev. 2003;7:622–640. doi: 10.1021/op0340311. - DOI
-
- Oberleitner N. Ressmann A. K. Bica K. Gärtner P. Fraaije M. W. Bornscheuer U. T. Rudroff F. Mihovilovic M. D. Green Chem. 2017;19:367–371. doi: 10.1039/C6GC01138A. - DOI
-
- Rudroff F. Mihovilovic M. D. Gröger H. Snajdrova R. Iding H. Bornscheuer U. T. Nat. Catal. 2018;1:12–22. doi: 10.1038/s41929-017-0010-4. - DOI
LinkOut - more resources
Full Text Sources
