Understanding AAV vector immunogenicity: from particle to patient
- PMID: 38323309
- PMCID: PMC10845199
- DOI: 10.7150/thno.89380
Understanding AAV vector immunogenicity: from particle to patient
Abstract
Gene therapy holds promise for patients with inherited monogenic disorders, cancer, and rare genetic diseases. Naturally occurring adeno-associated virus (AAV) offers a well-suited vehicle for clinical gene transfer due to its lack of significant clinical pathogenicity and amenability to be engineered to deliver therapeutic transgenes in a variety of cell types for long-term sustained expression. AAV has been bioengineered to produce recombinant AAV (rAAV) vectors for many gene therapies that are approved or in late-stage development. However, ongoing challenges hamper wider use of rAAV vector-mediated therapies. These include immunity against rAAV vectors, limited transgene packaging capacity, sub-optimal tissue transduction, potential risks of insertional mutagenesis and vector shedding. This review focuses on aspects of immunity against rAAV, mediated by anti-AAV neutralizing antibodies (NAbs) arising after natural exposure to AAVs or after rAAV vector administration. We provide an in-depth analysis of factors determining AAV seroprevalence and examine clinical approaches to managing anti-AAV NAbs pre- and post-vector administration. Methodologies used to quantify anti-AAV NAb levels and strategies to overcome pre-existing AAV immunity are also discussed. The broad adoption of rAAV vector-mediated gene therapies will require wider clinical appreciation of their current limitations and further research to mitigate their impact.
Keywords: humoral immunity; neutralizing antibodies; patient exclusion; redosing; seroprevalence.
© The author(s).
Conflict of interest statement
Competing Interests: Bijay P. Dhungel and John E. J. Rasko acknowledge support from Brandon Capital and Therapeutic Innovation Australia. John E. J. Rasko is supported by NHMRC (Investigator Grant 1177305 to him for “Driving clinical cell and gene therapy in Australia”), Cure the Future, and an anonymous foundation. He declares Supply of Material (MTA), consultancy, or honoraria with Bluebird Bio, Cynata, Gilead, Novartis, Pfizer, RareCyte, Roche, and SPARK Therapeutics; and shareholdings with RareCyte, Genea, and Woke. Ian Winburn, Candida da. da Fonseca Pereira, Kui Huang, and Amit Chhabra are employees of Pfizer and may own stock/options in the company.
Figures


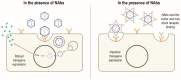

References
-
- Anguela XM, High KA. Entering the modern era of gene therapy. Annu Rev Med. 2019;70:273–88. - PubMed
-
- High KA, Roncarolo MG. Gene therapy. N Engl J Med. 2019;381(5):455–64. - PubMed
-
- Whitehead M, Osborne A, Yu-Wai-Man P, Martin K. Humoral immune responses to AAV gene therapy in the ocular compartment. Biol Rev Camb Philos Soc. 2021;96(4):1616–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

