Altered 5-HT2A/C receptor binding in the medulla oblongata in the sudden infant death syndrome (SIDS): Part II. Age-associated alterations in serotonin receptor binding profiles within medullary nuclei supporting cardiorespiratory homeostasis
- PMID: 38323418
- PMCID: PMC10880067
- DOI: 10.1093/jnen/nlae004
Altered 5-HT2A/C receptor binding in the medulla oblongata in the sudden infant death syndrome (SIDS): Part II. Age-associated alterations in serotonin receptor binding profiles within medullary nuclei supporting cardiorespiratory homeostasis
Abstract
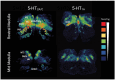
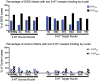
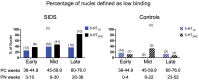
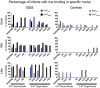
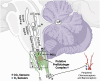
The failure of chemoreflexes, arousal, and/or autoresuscitation to asphyxia may underlie some sudden infant death syndrome (SIDS) cases. In Part I, we showed that some SIDS infants had altered 5-hydroxytryptamine (5-HT)2A/C receptor binding in medullary nuclei supporting chemoreflexes, arousal, and autoresuscitation. Here, using the same dataset, we tested the hypotheses that the prevalence of low 5-HT1A and/or 5-HT2A/C receptor binding (defined as levels below the 95% confidence interval of controls-a new approach), and the percentages of nuclei affected are greater in SIDS versus controls, and that the distribution of low binding varied with age of death. The prevalence and percentage of nuclei with low 5-HT1A and 5-HT2A/C binding in SIDS were twice that of controls. The percentage of nuclei with low 5-HT2A/C binding was greater in older SIDS infants. In >80% of older SIDS infants, low 5-HT2A/C binding characterized the hypoglossal nucleus, vagal dorsal nucleus, nucleus of solitary tract, and nuclei of the olivocerebellar subnetwork (important for blood pressure regulation). Together, our findings from SIDS infants and from animal models of serotonergic dysfunction suggest that some SIDS cases represent a serotonopathy. We present new hypotheses, yet to be tested, about how defects within serotonergic subnetworks may lead to SIDS.
Keywords: Autoradiography; Blood pressure recovery; Gasping; Hypoxia; Inferior olive; Nucleus of the solitary tract; Raphe.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Association of Neuropathologists, Inc.
Conflict of interest statement
The authors have no duality or conflicts of interest to declare.
Figures


References
-
- Goldstein RD, Blair PS, Sens MA, et al.; 3rd International Congress on Sudden Infant and Child Death. Inconsistent classification of unexplained sudden deaths in infants and children hinders surveillance, prevention and research: Recommendations from The 3rd International Congress on Sudden Infant and Child Death. Forensic Sci Med Pathol 2019;15:622–8 - PMC - PubMed
-
- Krous HF, Beckwith JB, Byard RW, et al.Sudden infant death syndrome and unclassified sudden infant deaths: A definitional and diagnostic approach. (Congresses). Pediatrics 2004;114:234–8 - PubMed
-
- Moon RY, Carlin RF, Hand I; TASK FORCE ON SUDDEN INFANT DEATH SYNDROME AND THE COMMITTEE ON FETUS AND NEWBORN. Sleep-related infant deaths: Updated 2022 recommendations for reducing infant deaths in the sleep environment. Pediatrics 2022;150:e2022057990. - PubMed
-
- Vennemann MM, Bajanowski T, Brinkmann B, et al.; GeSID Study Group. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics 2009;123:e406–10 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

