Pericyte Microvesicles as Plasma Biomarkers Reflecting Brain Microvascular Signaling in Patients With Acute Ischemic Stroke
- PMID: 38323422
- PMCID: PMC10896197
- DOI: 10.1161/STROKEAHA.123.045720
Pericyte Microvesicles as Plasma Biomarkers Reflecting Brain Microvascular Signaling in Patients With Acute Ischemic Stroke
Abstract
Background: Blood-based biomarkers have the potential to reflect cerebrovascular signaling after microvascular injury; yet, the detection of cell-specific signaling has proven challenging. Microvesicles retain parental cell surface antigens allowing detection of cell-specific signaling encoded in their cargo. In ischemic stroke, the progression of pathology involves changes in microvascular signaling whereby brain pericytes, perivascular cells wrapping the microcapillaries, are one of the early responders to the ischemic insult. Intercepting the pericyte signaling response peripherally by isolating pericyte-derived microvesicles may provide not only diagnostic information on microvascular injury but also enable monitoring of important pathophysiological mechanisms.
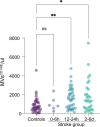
Methods: Plasma samples were collected from patients with acute ischemic stroke (n=39) at 3 time points after stroke onset: 0 to 6 hours, 12 to 24 hours, and 2 to 6 days, and compared with controls (n=39). Pericyte-derived microvesicles were isolated based on cluster of differentiation 140b expression and quantified by flow cytometry. The protein content was evaluated using a proximity extension assay, and vascular signaling pathways were examined using molecular signature hallmarks and gene ontology.
Results: In this case-control study, patients with acute ischemic stroke showed significantly increased numbers of pericyte-derived microvesicles (median, stroke versus controls) at 12 to 24 hours (1554 versus 660 microvesicles/μL; P=0.0041) and 2 to 6 days after stroke (1346 versus 660 microvesicles/μL; P=0.0237). Their proteome revealed anti-inflammatory properties mediated via downregulation of Kirsten rat sarcoma virus and IL (interleukin)-6/JAK/STAT3 signaling at 0 to 6 hours, but proangiogenic as well as proinflammatory signals at 12 to 24 hours. Between 2 and 6 days, proteins were mainly associated with vascular remodeling as indicated by activation of Hedgehog signaling in addition to proangiogenic signals.
Conclusions: We demonstrate that the plasma of patients with acute ischemic stroke reflects (1) an early and time-dependent increase of pericyte-derived microvesicles and (2) changes in the protein cargo of microvesicles over time indicating cell signaling specifically related to inflammation and vascular remodeling.
Keywords: microvesicles; pericytes; secretome; signaling; stroke.
Conflict of interest statement
Figures



References
-
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413 - PubMed
-
- Campbell BC, Mitchell PJ, Investigators E-I. Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2365–2366. doi: 10.1056/NEJMc1504715 - PubMed
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 - PubMed
-
- Gamez-Valero A, Beyer K, Borras FE. Extracellular vesicles, new actors in the search for biomarkers of dementias. Neurobiol Aging. 2019;74:15–20. doi: 10.1016/j.neurobiolaging.2018.10.006 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

