Integrated Stress Response Potentiates Ponatinib-Induced Cardiotoxicity
- PMID: 38323474
- PMCID: PMC10940206
- DOI: 10.1161/CIRCRESAHA.123.323683
Integrated Stress Response Potentiates Ponatinib-Induced Cardiotoxicity
Abstract
Background: Mitochondrial dysfunction is a primary driver of cardiac contractile failure; yet, the cross talk between mitochondrial energetics and signaling regulation remains obscure. Ponatinib, a tyrosine kinase inhibitor used to treat chronic myeloid leukemia, is among the most cardiotoxic tyrosine kinase inhibitors and causes mitochondrial dysfunction. Whether ponatinib-induced mitochondrial dysfunction triggers the integrated stress response (ISR) to induce ponatinib-induced cardiotoxicity remains to be determined.
Methods: Using human induced pluripotent stem cells-derived cardiomyocytes and a recently developed mouse model of ponatinib-induced cardiotoxicity, we performed proteomic analysis, molecular and biochemical assays to investigate the relationship between ponatinib-induced mitochondrial stress and ISR and their role in promoting ponatinib-induced cardiotoxicity.
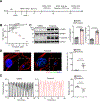
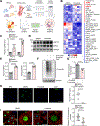
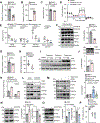
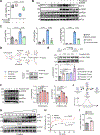
Results: Proteomic analysis revealed that ponatinib activated the ISR in cardiac cells. We identified GCN2 (general control nonderepressible 2) as the eIF2α (eukaryotic translation initiation factor 2α) kinase responsible for relaying mitochondrial stress signals to trigger the primary ISR effector-ATF4 (activating transcription factor 4), upon ponatinib exposure. Mechanistically, ponatinib treatment exerted inhibitory effects on ATP synthase activity and reduced its expression levels resulting in ATP deficits. Perturbed mitochondrial function resulting in ATP deficits then acts as a trigger of GCN2-mediated ISR activation, effects that were negated by nicotinamide mononucleotide, an NAD+ precursor, supplementation. Genetic inhibition of ATP synthase also activated GCN2. Interestingly, we showed that the decreased abundance of ATP also facilitated direct binding of ponatinib to GCN2, unexpectedly causing its activation most likely because of a conformational change in its structure. Importantly, administering an ISR inhibitor protected human induced pluripotent stem cell-derived cardiomyocytes against ponatinib. Ponatinib-treated mice also exhibited reduced cardiac function, effects that were attenuated upon systemic ISRIB administration. Importantly, ISRIB does not affect the antitumor effects of ponatinib in vitro.
Conclusions: Neutralizing ISR hyperactivation could prevent or reverse ponatinib-induced cardiotoxicity. The findings that compromised ATP production potentiates GCN2-mediated ISR activation have broad implications across various cardiac diseases. Our results also highlight an unanticipated role of ponatinib in causing direct activation of a kinase target despite its role as an ATP-competitive kinase inhibitor.
Keywords: GCN2; ISR; cardiotoxicity; mitochondria; ponatinib.
Conflict of interest statement
Figures


Comment in
-
Ponatinib-Induced Cardiomyocyte Toxicity: Dark Side of the Integrated Stress Response.Circ Res. 2024 Mar;134(5):502-504. doi: 10.1161/CIRCRESAHA.124.324164. Epub 2024 Feb 29. Circ Res. 2024. PMID: 38422183 Free PMC article. No abstract available.
References
-
- Global Burden of Disease Cancer C, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

