Organization of microtubule plus-end dynamics by phase separation in mitosis
- PMID: 38323478
- PMCID: PMC11337005
- DOI: 10.1093/jmcb/mjae006
Organization of microtubule plus-end dynamics by phase separation in mitosis
Abstract
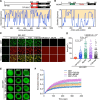
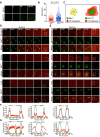
In eukaryotes, microtubule polymers are essential for cellular plasticity and fate decisions. End-binding (EB) proteins serve as scaffolds for orchestrating microtubule polymer dynamics and are essential for cellular dynamics and chromosome segregation in mitosis. Here, we show that EB1 forms molecular condensates with TIP150 and MCAK through liquid-liquid phase separation to compartmentalize the kinetochore-microtubule plus-end machinery, ensuring accurate kinetochore-microtubule interactions during chromosome segregation in mitosis. Perturbation of EB1-TIP150 polymer formation by a competing peptide prevents phase separation of the EB1-mediated complex and chromosome alignment at the metaphase equator in both cultured cells and Drosophila embryos. Lys220 of EB1 is dynamically acetylated by p300/CBP-associated factor in early mitosis, and persistent acetylation at Lys220 attenuates phase separation of the EB1-mediated complex, dissolves droplets in vitro, and harnesses accurate chromosome segregation. Our data suggest a novel framework for understanding the organization and regulation of eukaryotic spindle for accurate chromosome segregation in mitosis.
Keywords: EB1; acetylation; microtubule dynamics; mitosis; phase separation.
© The Author(s) (2024). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures


References
-
- Akhmanova A., Steinmetz M.O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309–322. - PubMed
-
- Akhmanova A., Steinmetz M.O. (2015). Control of microtubule organization and dynamics: two ends in the limelight. Nat. Rev. Mol. Cell Biol. 16, 711–726. - PubMed
-
- Bergeron-Sandoval L.P., Safaee N., Michnick S.W. (2016). Mechanisms and consequences of macromolecular phase separation. Cell 165, 1067–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

