Investigating alpha-synuclein co-pathology in Alzheimer's disease by means of cerebrospinal fluid alpha-synuclein seed amplification assay
- PMID: 38323747
- PMCID: PMC11032521
- DOI: 10.1002/alz.13658
Investigating alpha-synuclein co-pathology in Alzheimer's disease by means of cerebrospinal fluid alpha-synuclein seed amplification assay
Abstract
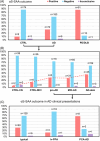
Introduction: Lewy body disease, a frequently observed co-pathology in Alzheimer's disease (AD), can be identified antemortem in cerebrospinal fluid (CSF) by α-synuclein seed amplification assay (αS-SAA). The prevalence and clinical impact of CSF αS-SAA positivity in AD are still unknown.
Methods: αS-SAA was performed on CSF samples from 240 AD patients (preclinical, prodromal, and dementia stages), 85 controls, 84 patients with Parkinson's disease (PD), and 21 patients with PD with dementia or dementia with Lewy bodies. In AD patients, associations between αS-SAA positivity and cognitive changes were also evaluated.
Results: In agreement with available neuropathological studies, αS-SAA positivity was observed in 30% of AD patients (vs 9% in controls), and was associated with cognitive decline, visuospatial impairment, and behavioral disturbances.
Discussion: αS-SAA positivity in AD patients reflects the prevalence observed in neuropathological series and is associated with a worse clinical outcome. These data confirm the validity of CSF αS-SAA positivity as biomarker of synucleinopathy.
Keywords: Alzheimer's disease; biomarkers; cerebrospinal fluid; neuropsychological evaluation; seed amplification assay; synucleinopathy.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare the following competing financial interests: Prof. Parnetti served as an Advisory Board Member for Fujirebio, IBL, Roche, and Merck. Dr. Concha, Ms. Farris, and Mr. Ma are inventors of several patents related to SAA technology (PMCA) and are associated with Amprion Inc., a biotech company focused on the commercial utilization of SAA for diagnosis. All other authors declare no financial and non‐financial competing interests. Author disclosures are available in the supporting information.
Figures


References
-
- Siderowf A, Concha‐Marambio L, Lafontant DE, et al. Assessment of heterogeneity among participants in the Parkinson's progression markers initiative cohort using α‐synuclein seed amplification: a cross‐sectional study. Lancet Neurol. 2023;22(5):407‐417. doi:10.1016/S1474-4422(23)00109-6 - DOI - PMC - PubMed

