An obligate microsporidian parasite modulates defense against opportunistic bacterial infection in the yellow fever mosquito , Aedes aegypti
- PMID: 38323845
- PMCID: PMC10900900
- DOI: 10.1128/msphere.00678-23
An obligate microsporidian parasite modulates defense against opportunistic bacterial infection in the yellow fever mosquito , Aedes aegypti
Abstract
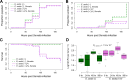
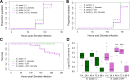
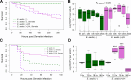
The ability of Aedes aegypti mosquitoes to transmit vertebrate pathogens depends on multiple factors, including the mosquitoes' life history traits, immune response, and microbiota (i.e., the microbes associated with the mosquito throughout its life). The microsporidium Edhazardia aedis is an obligate intracellular parasite that specifically infects Ae. aegypti mosquitoes and severely affects mosquito survival and other life history traits critical for pathogen transmission. In this work, we investigated how E. aedis impacts bacterial infection with Serratia marcescens in Ae. aegypti mosquitoes. We measured development, survival, and bacterial load in both larval and adult stages of mosquitoes. In larvae, E. aedis exposure was either horizontal or vertical and S. marcescens was introduced orally. Regardless of the route of transmission, E. aedis exposure resulted in significantly higher S. marcescens loads in larvae. E. aedis exposure also significantly reduced larval survival but subsequent exposure to S. marcescens had no effect. In adult females, E. aedis exposure was only horizontal and S. marcescens was introduced orally or via intrathoracic injection. In both cases, E. aedis infection significantly increased S. marcescens bacterial loads in adult female mosquitoes. In addition, females infected with E. aedis and subsequently injected with S. marcescens suffered 100% mortality which corresponded with a rapid increase in bacterial load. These findings suggest that exposure to E. aedis can influence the establishment and/or replication of other microbes in the mosquito. This has implications for understanding the ecology of mosquito immune defense and potentially disease transmission by mosquito vector species.
Importance: The microsporidium Edhazardia aedis is a parasite of the yellow fever mosquito, Aedes aegypti. This mosquito transmits multiple viruses to humans in the United States and around the world, including dengue, yellow fever, and Zika viruses. Hundreds of millions of people worldwide will become infected with one of these viruses each year. E. aedis infection significantly reduces the lifespan of Ae. aegypti and is therefore a promising novel biocontrol agent. Here, we show that when the mosquito is infected with this parasite, it is also significantly more susceptible to infection by an opportunistic bacterial pathogen, Serratia marcescens. This novel discovery suggests the mosquito's ability to control infection by other microbes is impacted by the presence of the parasite.
Keywords: Aedes aegypti; Edhazardia aedis; Serratia marcescens; horizontal transmission; immune defense; immune response; immunity; microsporidia; parasite; vertical transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Centers for disease control and prevention, “mosquito-borne diseases". 2016. www.cdc.gov/niosh/topics/outdoor/mosquito-borne/default.html.
-
- World health organization . 2023. Dengue and severe dengue. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
