Tenapanor as Therapy for Hyperphosphatemia in Maintenance Dialysis Patients: Results from the OPTIMIZE Study
- PMID: 38323855
- PMCID: PMC11146652
- DOI: 10.34067/KID.0000000000000387
Tenapanor as Therapy for Hyperphosphatemia in Maintenance Dialysis Patients: Results from the OPTIMIZE Study
Abstract
Key Points:
Tenapanor, a first-in-class local inhibitor of sodium/hydrogen exchanger isoform 3, acts as a phosphate absorption inhibitor by decreasing paracellular phosphate absorption.
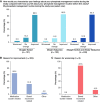
Tenapanor alone or with phosphate binders achieved P ≤ 5.5 mg/dl over 10 weeks in 34%–38% of patients taking phosphate binders at baseline.
Tenapanor can help adults with CKD on maintenance dialysis achieve normal serum phosphate concentrations.
Background: OPTIMIZE was a randomized, open-label study evaluating different tenapanor initiation methods. OPTIMIZE evaluated tenapanor alone and in combination with phosphate binders (PBs) to achieve target serum phosphate (P) ≤5.5 mg/dl.
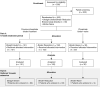
Methods: Patients with inadequately controlled P receiving maintenance dialysis from 42 US locations who were taking PBs with baseline P > 5.5 mg/dl and ≤ 10.0 mg/dl, or were PB-naive with baseline P > 4.5 mg/dl and ≤ 10.0 mg/dl, were included in OPTIMIZE. Participants taking PBs at baseline were randomized to switch from PBs to tenapanor (Straight Switch; n=151) or reduce PB dosage by ≥50% and add tenapanor (Binder Reduction; n=152); PB-naive patients started tenapanor alone (Binder-Naive; n=30). Participants received tenapanor 30 mg twice a day for 10 weeks (part A), followed by an elective, 16-week open-label extension (part B). Outcomes included changes from baseline in P, intact fibroblast growth factor 23, parathyroid hormone, serum calcium, and medication burden; patient-reported outcomes; and safety.
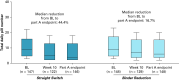
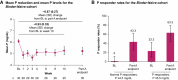
Results: By part A end point, 34.4% (Straight Switch), 38.2% (Binder Reduction), and 63.3% (Binder-Naive) of patients achieved P ≤ 5.5 mg/dl. Mean P reduction and median pill burden reduction from baseline to part A end point were 0.91±1.7 mg/dl and 4 pills/d for the Straight Switch and 0.99±1.8 mg/dl and 1 pill/d for the Binder Reduction group. The mean P reduction for Binder-Naive patients was 0.87±1.5 mg/dl. Among Straight Switch and Binder Reduction patients who completed patient experience questionnaires, 205 of 243 (84.4%) reported an improved phosphate management routine. Diarrhea was the most common adverse event (133 of 333 [39.9%]).
Conclusions: Tenapanor as monotherapy or in combination with PBs effectively lowered P toward the target range in patients who were PB-naive or who were not at goal despite PB use.
Clinical Trial registration number:
Trial registration: ClinicalTrials.gov NCT02081534 NCT02675998 NCT03427125 NCT03824587 NCT03988920 NCT04549597.
Conflict of interest statement
G.A. Block reports the following: Employer: US Renal Care; Ownership Interest: Ardelyx, Inc. and US Renal Care, Inc.; Research Funding: Akebia; and Advisory or Leadership Role: Ardelyx, Inc.. S. Edelstein reports the following: Employer: Ardelyx, Inc.; Ownership Interest: Ardelyx, Inc.; Research Funding: Ardelyx, Inc.; Patents or Royalties: Ardelyx, Inc.; and Advisory or Leadership Role: Ardelyx, Inc.. G. Fadda reports the following: Employer: Balboa Nephrology Medical Group; Research Funding: AstraZeneca, Calliditas, and GSK; Honoraria: AstraZeneca, Calliditas, and GSK; and Speakers Bureau: AstraZeneca, Bayer, Calliditas, and GSK. S.Z. Fadem reports the following: Employer: Kidney Associates, PLLC; Ownership Interest: Apple—few shares; DaVita—few shares; and The rest—money managers; Research Funding: I am a PI and do research for Reata, Ardelyx, Inc. through DaVita Clinical Research; Patents or Royalties: Have patent on encrypted software—no royalties yet; Advisory or Leadership Role: AAKP Chair Medical Advisory Board and Co-editor of aakpRenalife; NKF Serving Texas—Chair Medical Advisory Board; and Other Interests or Relationships: Own Touchcalc, Founded Nephron Information Center. S. Fishbane reports the following: Employer: Northwell Health; Consultancy: Aredelyx, Inc., AstraZeneca, Cara Therapeutics, Galderma, GSK, and Vifor; Research Funding: AstraZeneca, Galderma, Otsuka, and Vertex; and Honoraria: Ardelyx, Inc., AstraZeneca, GSK, and Vifor. R.I. Lynn reports the following: Employer: Kidney Medical Associates. L. Pagliaro reports the following: Employer: Ardelyx, Inc.. P.E. Pergola reports the following: Employer: Renal Associates, P.A.; Consultancy: Ardelyx, Inc., AstraZeneca, Bayer, Calico, Furoscix, GSK, Lilac, Novo Nordisk, Renibus, and Unicycive; Ownership Interest: Unicycive Therapeutics; Research Funding: Prinicipal or subinvestigator on multiple clinical trials. The contracts are with my practice, not individual; and Advisory or Leadership Role: Ardelyx, Inc. and Unicycive. D.P. Rosenbaum reports the following: Employer: Ardelyx, Inc.; Ownership Interest: Ardelyx, Inc.; and Advisory or Leadership Role: Ardelyx, Inc.. A.L. Silva reports the following: Employer: Boise Kidney & Hypertension Institute; Consultancy: Aurinia, Boehringer-Ingelheim, GSK, Novartis, ProKidney, Reata Pharmaceuticals, and Travere; Research Funding: Akebia, Ardelyx, Inc., AstraZeneca, Cara, Cincor, Diamedica, Gilead, Goldfinch Bio, GSK, Mineralys, Novartis, OPKO Renal, ProKidney, Reata Pharmaceuticals, Regulus, Takeda, and Travere; Advisory or Leadership Role: Ardelyx, Inc., Aurinia, Boehringer Ingelheim, GSK, Novartis, ProKidney, Reata, and Travere; and Speakers Bureau: Amgen, AstraZeneca, Aurinia, Bayer, Boehringer-Ingelheim, Janssen, OPKO Renal, and Vifor. D.M. Spiegel reports the following: Employer: Ardelyx, Inc.; Ownership Interest: Stock grants and options from Ardelyx, Inc.; and Other Interests or Relationships: Member: ASN, ISN, and NKF. S.M. Sprague reports the following: Employer: NorthShore University Health System and University of Chicago Pritzker School of Medicine; Consultancy: Amgen, Ardelyx, Inc., Bayer, Fresenius, Horizon, Litholink Corp, OPKO, Shire, and Vifor; Ownership Interest: Individually owned stocks; Apple, Baxter, Bristol Myers, Coca Cola, First Australia Fund, IBM, and Walgreens; Research Funding: Amgen, Amylot, Ardelyx, Inc., OPKO, Reata, and Takeda; Honoraria: Amgen, Ardelyx, Inc., Bayer, Fresenius, OPKO, and Vifor; Advisory or Leadership Role: American Association of Endocrine Surgeons,
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

