Uniaxial mechanical stretch properties correlated with three-dimensional microstructure of human dermal skin
- PMID: 38324073
- PMCID: PMC11101527
- DOI: 10.1007/s10237-023-01813-3
Uniaxial mechanical stretch properties correlated with three-dimensional microstructure of human dermal skin
Abstract
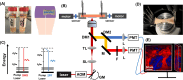
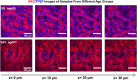
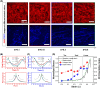
The intact and healthy skin forms a barrier to the outside world and protects the body from mechanical impact. The skin is a complex structure with unique mechano-elastic properties. To better direct the design of biomimetic materials and induce skin regeneration in wounds with optimal outcome, more insight is required in how the mechano-elastic properties emerge from the skin's main constituents, collagen and elastin fibers. Here, we employed two-photon excited autofluorescence and second harmonic generation microscopy to characterize collagen and elastin fibers in 3D in 24 human dermis skin samples. Through uniaxial stretching experiments, we derive uni-directional mechanical properties from resultant stress-strain curves, including the initial Young's modulus, elastic Young's modulus, maximal stress, and maximal and mid-strain values. The stress-strain curves show a large variation, with an average Young's modules in the toe and linear regions of 0.1 MPa and 21 MPa. We performed a comprehensive analysis of the correlation between the key mechanical properties with age and with microstructural parameters, e.g., fiber density, thickness, and orientation. Age was found to correlate negatively with Young's modulus and collagen density. Moreover, real-time monitoring during uniaxial stretching allowed us to observe changes in collagen and elastin alignment. Elastin fibers aligned significantly in both the heel and linear regions, and the collagen bundles engaged and oriented mainly in the linear region. This research advances our understanding of skin biomechanics and yields input for future first principles full modeling of skin tissue.
Keywords: Collagen fibers; Elastin fibers; Human skin; Mechanical properties; Second harmonic generation; Uniaxial skin stretch.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures





References
-
- Allain Jean-Marc, Lynch Barbara, Schanne-Klein Marie-Claire (2019) Multiscale characterisation of skin mechanics through in situ imaging. Skin Biophys From Exp Charact Adv Modell pp. 235–263
-
- Bancelin Stéphane, Lynch Barbara, Bonod-Bidaud Christelle, Ducourthial Guillaume, Psilodimitrakopoulos Sotiris, Dokládal Petr, Allain Jean-Marc, Schanne-Klein Marie-Claire, Ruggiero Florence. Ex vivo multiscale quantitation of skin biomechanics in wild-type and genetically-modified mice using multiphoton microscopy. Sci Rep. 2015;5(1):17635. doi: 10.1038/srep17635. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

