In-label, off-label prescription, efficacy and tolerability of dalbavancin: report from a National Registry
- PMID: 38324144
- PMCID: PMC11289212
- DOI: 10.1007/s15010-024-02176-2
In-label, off-label prescription, efficacy and tolerability of dalbavancin: report from a National Registry
Abstract
Purpose: Although dalbavancin is currently approved for the treatment of ABSSIs, several studies suggest its efficacy and tolerance as long-term therapy for other off-label indications requiring prolonged intravenous antibiotic administration.
Methods: We conducted a prospective nationwide study of dalbavancin use in real-life settings for both approved and off-label indications analysing for each case the clinical and microbiological characteristics of infection the efficacy and safety of treatments.
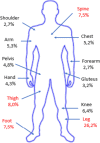
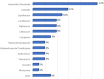
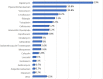
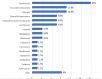
Results: During the study period (from December 2018 to July 2021), the ID specialists from 14 different centres enrolled 223 patients treated with dalbavancin [141 males (63%) and 82 females (37%); male/female ratio 1.72; mean age 59 (SD 17.2) years, (range 15-96). Most patients in the study population (136/223; 61.0%) came from community rather than health care facilities and most of them were visited in Infectious Diseases wards (93/223; 41.7%) and clinics (55/223; 24.7%) even though some patients were cured in other settings, such as surgery wards (18/223; 8.1%), orthopaedic wards (11/223; 4.9%), Emergency Rooms (7/223; 3.1%) and non-surgical other than ID wards (6/223; 2.7%). The most common ID diagnoses were osteomyelitis (44 cases/223; 19.7%; of which 29 acute and 15 chronic osteomyelitis), cellulitis (28/223; 12.5%), cutaneous abscess (23/223; 10.3%), orthopaedic prosthesis-associated infection (22/223; 9.9%), surgical site infection (20/223; 9.0%) and septic arthritis (15/223; 6.7%).
Conclusion: In conclusion, by virtue of its PK/PD properties, dalbavancin represents a valuable option to daily in-hospital intravenous or outpatient antimicrobial regimens also for off-label indications requiring a long-term treatment of Gram-positive infections.
Keywords: Dalbavancin; In-label; Off-label.
© 2024. The Author(s).
Conflict of interest statement
None to declare.
Figures
Similar articles
-
Multicenter clinical experience of real life Dalbavancin use in gram-positive infections.Int J Infect Dis. 2019 Apr;81:210-214. doi: 10.1016/j.ijid.2019.02.013. Epub 2019 Feb 19. Int J Infect Dis. 2019. PMID: 30794940 Clinical Trial.
-
Real-World Use of Dalbavancin in the Era of Empowerment of Outpatient Antimicrobial Treatment: A Careful Appraisal Beyond Approved Indications Focusing on Unmet Clinical Needs.Drug Des Devel Ther. 2021 Aug 3;15:3349-3378. doi: 10.2147/DDDT.S313756. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34376971 Free PMC article. Review.
-
Clinical use of dalbavancin in the Italian SUSANA cohort (SUrveillance of SAfety and outcome of New Antibiotics).New Microbiol. 2025 May;48(1):35-45. New Microbiol. 2025. PMID: 40314680
-
Real-life experience with IV dalbavancin in Canada; results from the CLEAR (Canadian LEadership on Antimicrobial Real-life usage) registry.J Glob Antimicrob Resist. 2024 Sep;38:154-157. doi: 10.1016/j.jgar.2024.06.002. Epub 2024 Jun 20. J Glob Antimicrob Resist. 2024. PMID: 38908823
-
Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: A systematic review.Int J Antimicrob Agents. 2020 Sep;56(3):106069. doi: 10.1016/j.ijantimicag.2020.106069. Epub 2020 Jun 27. Int J Antimicrob Agents. 2020. PMID: 32603683
Cited by
-
Proactive Therapeutic Drug MONiToring to Guide Suppressive Antibiotic Therapy with DALBAvaNcin ( > 12 weeks) in Osteoarticular Infections (MONTALBANO).J Bone Jt Infect. 2025 Jul 30;10(4):255-263. doi: 10.5194/jbji-10-255-2025. eCollection 2025. J Bone Jt Infect. 2025. PMID: 40746661 Free PMC article.
-
Bridging the Gap: A Systematic Review with Expert Opinion on the Use of Dalbavancin for In-Label and Off-Label Indications in Pediatric Patients.Antibiotics (Basel). 2025 Jan 23;14(2):121. doi: 10.3390/antibiotics14020121. Antibiotics (Basel). 2025. PMID: 40001365 Free PMC article. Review.
-
What Place Is There for Long-Acting Antibiotics in the Management of Gram-Positive Infections? A Qualitative Cross-Sectional Study.Antibiotics (Basel). 2024 Jul 12;13(7):644. doi: 10.3390/antibiotics13070644. Antibiotics (Basel). 2024. PMID: 39061326 Free PMC article.
-
Multidose Dalbavancin Population Pharmacokinetic Analysis for Prolonged Target Attainment in Patients Requiring Long-Term Treatment.Antibiotics (Basel). 2025 Feb 13;14(2):190. doi: 10.3390/antibiotics14020190. Antibiotics (Basel). 2025. PMID: 40001433 Free PMC article.
-
Dalbavancin for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in pediatric patients: a case series.Infez Med. 2024 Jun 1;32(2):231-240. doi: 10.53854/liim-3202-11. eCollection 2024. Infez Med. 2024. PMID: 38827834 Free PMC article.
References
-
- Esposito S, Noviello S, Leone S. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Infez Med. 2015;23:313–7. - PubMed
-
- Palmieri F, Alberici F, Deales A, et al. Early discharge of infectious disease patients: an opportunity or extra cost for the Italian Healthcare System? Infez Med. 2013;21:270–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical