Effects of exogenous lactate on lipid, protein, and glucose metabolism-a randomized crossover trial in healthy males
- PMID: 38324259
- PMCID: PMC11193511
- DOI: 10.1152/ajpendo.00301.2023
Effects of exogenous lactate on lipid, protein, and glucose metabolism-a randomized crossover trial in healthy males
Abstract
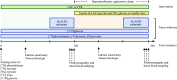
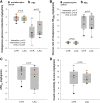
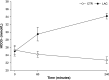
Lactate may inhibit lipolysis and thus enhance insulin sensitivity, but there is a lack of metabolic human studies. This study aimed to determine how hyperlactatemia affects lipolysis, glucose- and protein metabolism, and insulin sensitivity in healthy men. In a single-blind, randomized, crossover design, eight healthy men were studied after an overnight fast on two occasions: 1) during a sodium-lactate infusion (LAC) and 2) during a sodium-matched NaCl infusion (CTR). Both days consisted of a 3-h postabsorptive period followed by a 3-h hyperinsulinemic-euglycemic clamp (HEC). Lipolysis rate, endogenous glucose production (EGP), and delta glucose rate of disappearance (ΔRdglu) were evaluated using [9,10-3H]palmitate and [3-3H]glucose tracers. In addition, whole body- and forearm protein metabolism was assessed using [15N]phenylalanine, [2H4]tyrosine, [15N]tyrosine, and [13C]urea tracers. In the postabsorptive period, plasma lactate increased to 2.7 ± 0.5 mmol/L during LAC vs. 0.6 ± 0.3 mmol/L during CTR (P < 0.001). In the postabsorptive period, palmitate flux was 30% lower during LAC compared with CTR (84 ± 32 µmol/min vs. 120 ± 35 µmol/min, P = 0.003). During the HEC, palmitate flux was suppressed similarly during both interventions (P = 0.7). EGP, ΔRdglu, and M value were similar during LAC and CTR. During HEC, LAC increased whole body phenylalanine flux (P = 0.02) and protein synthesis (P = 0.03) compared with CTR; LAC did not affect forearm protein metabolism compared with CTR. Lactate infusion inhibited lipolysis by 30% under postabsorptive conditions but did not affect glucose metabolism or improve insulin sensitivity. In addition, whole body phenylalanine flux was increased. Clinical trial registrations: NCT04710875.NEW & NOTEWORTHY Lactate is a decisive intermediary metabolite, serving as an energy substrate and a signaling molecule. The present study examines the effects of lactate on substrate metabolism and insulin sensitivity in healthy males. Hyperlactatemia reduces lipolysis by 30% without affecting insulin sensitivity and glucose metabolism. In addition, hyperlactatemia increases whole body amino acid turnover rate.
Keywords: energy expenditure; insulin sensitivity; lactate; lipolysis; protein metabolism.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
Effects of adrenaline on lactate, glucose, lipid and protein metabolism in the placebo controlled bilaterally perfused human leg.Acta Physiol (Oxf). 2011 Aug;202(4):641-8. doi: 10.1111/j.1748-1716.2011.02316.x. Epub 2011 May 27. Acta Physiol (Oxf). 2011. PMID: 21624100 Clinical Trial.
-
Intact pituitary function is decisive for the catabolic response to TNF-α: studies of protein, glucose and fatty acid metabolism in hypopituitary and healthy subjects.J Clin Endocrinol Metab. 2015 Feb;100(2):578-86. doi: 10.1210/jc.2014-2489. Epub 2014 Nov 6. J Clin Endocrinol Metab. 2015. PMID: 25375979 Clinical Trial.
-
A model mimicking catabolic inflammatory disease; a controlled randomized study in humans.PLoS One. 2020 Nov 5;15(11):e0241274. doi: 10.1371/journal.pone.0241274. eCollection 2020. PLoS One. 2020. PMID: 33151986 Free PMC article. Clinical Trial.
-
Metabolic and thermogenic effects of lactate infusion in humans.Am J Physiol. 1993 Sep;265(3 Pt 1):E504-12. doi: 10.1152/ajpendo.1993.265.3.E504. Am J Physiol. 1993. PMID: 8214058
-
Acute effects of ghrelin administration on glucose and lipid metabolism.J Clin Endocrinol Metab. 2008 Feb;93(2):438-44. doi: 10.1210/jc.2007-2018. Epub 2007 Nov 27. J Clin Endocrinol Metab. 2008. PMID: 18042651 Clinical Trial.
Cited by
-
Cardiovascular effects of lactate in healthy adults.Crit Care. 2025 Jan 17;29(1):30. doi: 10.1186/s13054-025-05259-0. Crit Care. 2025. PMID: 39825426 Free PMC article. Clinical Trial.
-
Preparation and Preclinical Characterization of a Simple Ester for Dual Exogenous Supply of Lactate and Beta-hydroxybutyrate.J Agric Food Chem. 2024 Sep 11;72(36):19883-19890. doi: 10.1021/acs.jafc.4c04849. Epub 2024 Aug 30. J Agric Food Chem. 2024. PMID: 39214666 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

