Parameter Space and Potential for Biomarker Development in 25 Years of fMRI Drug Cue Reactivity: A Systematic Review
- PMID: 38324323
- PMCID: PMC11304510
- DOI: 10.1001/jamapsychiatry.2023.5483
Parameter Space and Potential for Biomarker Development in 25 Years of fMRI Drug Cue Reactivity: A Systematic Review
Abstract
Importance: In the last 25 years, functional magnetic resonance imaging drug cue reactivity (FDCR) studies have characterized some core aspects in the neurobiology of drug addiction. However, no FDCR-derived biomarkers have been approved for treatment development or clinical adoption. Traversing this translational gap requires a systematic assessment of the FDCR literature evidence, its heterogeneity, and an evaluation of possible clinical uses of FDCR-derived biomarkers.
Objective: To summarize the state of the field of FDCR, assess their potential for biomarker development, and outline a clear process for biomarker qualification to guide future research and validation efforts.
Evidence review: The PubMed and Medline databases were searched for every original FDCR investigation published from database inception until December 2022. Collected data covered study design, participant characteristics, FDCR task design, and whether each study provided evidence that might potentially help develop susceptibility, diagnostic, response, prognostic, predictive, or severity biomarkers for 1 or more addictive disorders.
Findings: There were 415 FDCR studies published between 1998 and 2022. Most focused on nicotine (122 [29.6%]), alcohol (120 [29.2%]), or cocaine (46 [11.1%]), and most used visual cues (354 [85.3%]). Together, these studies recruited 19 311 participants, including 13 812 individuals with past or current substance use disorders. Most studies could potentially support biomarker development, including diagnostic (143 [32.7%]), treatment response (141 [32.3%]), severity (84 [19.2%]), prognostic (30 [6.9%]), predictive (25 [5.7%]), monitoring (12 [2.7%]), and susceptibility (2 [0.5%]) biomarkers. A total of 155 interventional studies used FDCR, mostly to investigate pharmacological (67 [43.2%]) or cognitive/behavioral (51 [32.9%]) interventions; 141 studies used FDCR as a response measure, of which 125 (88.7%) reported significant interventional FDCR alterations; and 25 studies used FDCR as an intervention outcome predictor, with 24 (96%) finding significant associations between FDCR markers and treatment outcomes.
Conclusions and relevance: Based on this systematic review and the proposed biomarker development framework, there is a pathway for the development and regulatory qualification of FDCR-based biomarkers of addiction and recovery. Further validation could support the use of FDCR-derived measures, potentially accelerating treatment development and improving diagnostic, prognostic, and predictive clinical judgments.
Conflict of interest statement
Dr Schacht reported nonfinancial support from Bausch Health outside the submitted work. Dr Bach reported grants from German Research Foundation and German Ministry of Science and Education outside the submitted work. Dr Childress reported grants from the National Institute on Drug Abuse during the conduct of the study. Dr Filbey reported grants from the National Institutes of Health during the conduct of the study. Dr Hanlon reported owning stock in BrainsWay outside the submitted work. Dr Heilig reported grants and materials from BrainsWay and Janssen as well as personal fees from Aelis Farma, Indivior, and Camurus outside the submitted work. Dr Joseph reported grants from the National Institute on Drug Abuse during the conduct of the study. Dr Kaufman reported grants from the National Institutes of Health during the conduct of the study and has patent US9737562B2 issued and patent EU2931291B1 issued. Dr Oliver has patent 11308325 issued. Dr Steele reported grants from the National Institutes of Health during the conduct of the study. Dr Vollstädt-Klein reported grants from the Central Institute of Mental Health German Research Foundation during the conduct of the study. Dr Wetherill reported grants from the National Institutes of Health outside the submitted work. Dr Verdejo-Garcia reported personal fees from Servier, Elsevier, and Springer-Nature outside the submitted work. Dr Potenza reported grants from the National Institutes of Health during the conduct of the study; personal fees from Opiant Pharmaceuticals, Idorsia Pharmaceuticals, Baria-Tek, Game Day Data, and Addiction Policy Forum as well as research support from Mohegan Sun Casino, Children and Screens, and Connecticut Council on Problem Gambling outside the submitted work; patent application support from Novartis; and is a board member for the International Society of Addiction Medicine, Addiction Policy Forum, and National Council on Problem Gambling. No other disclosures were reported.
Figures

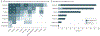

References
Publication types
MeSH terms
Substances
Grants and funding
- R34 AT008948/AT/NCCIH NIH HHS/United States
- R01 DA047851/DA/NIDA NIH HHS/United States
- R01 AT010627/AT/NCCIH NIH HHS/United States
- R01 DA048301/DA/NIDA NIH HHS/United States
- UG3 DA048388/DA/NIDA NIH HHS/United States
- R03 DA052719/DA/NIDA NIH HHS/United States
- K01 AA029712/AA/NIAAA NIH HHS/United States
- R21 DA045853/DA/NIDA NIH HHS/United States
- R01 DA041866/DA/NIDA NIH HHS/United States
- R01 DA042490/DA/NIDA NIH HHS/United States
- R03 DA029163/DA/NIDA NIH HHS/United States
- R21 AA030284/AA/NIAAA NIH HHS/United States
- R01 AA029611/AA/NIAAA NIH HHS/United States
- R01 DA041528/DA/NIDA NIH HHS/United States
- R01 DA055774/DA/NIDA NIH HHS/United States
- R01 AA026859/AA/NIAAA NIH HHS/United States
- R61 AT009337/AT/NCCIH NIH HHS/United States
- R01 AT007922/AT/NCCIH NIH HHS/United States
- R01 AA027765/AA/NIAAA NIH HHS/United States
- R01 AA023665/AA/NIAAA NIH HHS/United States
- RF1 MH128614/MH/NIMH NIH HHS/United States
- P50 DA009241/DA/NIDA NIH HHS/United States
- R21 CA184254/CA/NCI NIH HHS/United States
- K23 AA028535/AA/NIAAA NIH HHS/United States
- R37 DA044467/DA/NIDA NIH HHS/United States
- UG1 DA050207/DA/NIDA NIH HHS/United States
- R41 MH118130/MH/NIMH NIH HHS/United States
- K12 DA000167/DA/NIDA NIH HHS/United States
- R01 DA053342/DA/NIDA NIH HHS/United States
- R21 AG062004/AG/NIA NIH HHS/United States
- R01 DA047119/DA/NIDA NIH HHS/United States
- P50 AA010761/AA/NIAAA NIH HHS/United States
- K24 AA030788/AA/NIAAA NIH HHS/United States
- R01 DA049547/DA/NIDA NIH HHS/United States
- R01 AA029406/AA/NIAAA NIH HHS/United States
- R01 AA025365/AA/NIAAA NIH HHS/United States
- R21 DA054281/DA/NIDA NIH HHS/United States
- R34 DA037886/DA/NIDA NIH HHS/United States
- R01 DK121551/DK/NIDDK NIH HHS/United States
- R01 DA054275/DA/NIDA NIH HHS/United States
- K23 DA042898/DA/NIDA NIH HHS/United States
- R01 DA045162/DA/NIDA NIH HHS/United States

