Seed longevity and genome damage
- PMID: 38324350
- PMCID: PMC11111285
- DOI: 10.1042/BSR20230809
Seed longevity and genome damage
Abstract
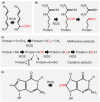
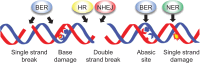
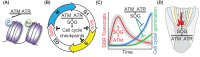
Seeds are the mode of propagation for most plant species and form the basis of both agriculture and ecosystems. Desiccation tolerant seeds, representative of most crop species, can survive maturation drying to become metabolically quiescent. The desiccated state prolongs embryo viability and provides protection from adverse environmental conditions, including seasonal periods of drought and freezing often encountered in temperate regions. However, the capacity of the seed to germinate declines over time and culminates in the loss of seed viability. The relationship between environmental conditions (temperature and humidity) and the rate of seed deterioration (ageing) is well defined, but less is known about the biochemical and genetic factors that determine seed longevity. This review will highlight recent advances in our knowledge that provide insight into the cellular stresses and protective mechanisms that promote seed survival, with a focus on the roles of DNA repair and response mechanisms. Collectively, these pathways function to maintain the germination potential of seeds. Understanding the molecular basis of seed longevity provides important new genetic targets for the production of crops with enhanced resilience to changing climates and knowledge important for the preservation of plant germplasm in seedbanks.
Keywords: DNA repair; Seed; genome stability; germination; mutation; recombination.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

