Aligned cryogel fibers incorporated 3D printed scaffold effectively facilitates bone regeneration by enhancing cell recruitment and function
- PMID: 38324693
- PMCID: PMC10849600
- DOI: 10.1126/sciadv.adk6722
Aligned cryogel fibers incorporated 3D printed scaffold effectively facilitates bone regeneration by enhancing cell recruitment and function
Abstract
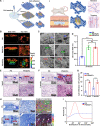
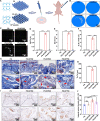
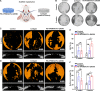
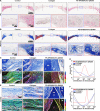
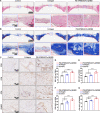
Reconstructing extensive cranial defects represents a persistent clinical challenge. Here, we reported a hybrid three-dimensional (3D) printed scaffold with modification of QK peptide and KP peptide for effectively promoting endogenous cranial bone regeneration. The hybrid 3D printed scaffold consists of vertically aligned cryogel fibers that guide and promote cell penetration into the defect area in the early stages of bone repair. Then, the conjugated QK peptide and KP peptide further regulate the function of the recruited cells to promote vascularization and osteogenic differentiation in the defect area. The regenerated bone volume and surface coverage of the dual peptide-modified hybrid scaffold were significantly higher than the positive control group. In addition, the dual peptide-modified hybrid scaffold demonstrated sustained enhancement of bone regeneration and avoidance of bone resorption compared to the collagen sponge group. We expect that the design of dual peptide-modified hybrid scaffold will provide a promising strategy for bone regeneration.
Figures



References
-
- Berretta S., Evans K., Ghita O., Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 139, 141–152 (2018).
-
- Takizawa T., Nakayama N., Haniu H., Aoki K., Okamoto M., Nomura H., Tanaka M., Sobajima A., Yoshida K., Kamanaka T., Ajima K., Oishi A., Kuroda C., Ishida H., Okano S., Kobayashi S., Kato H., Saito N., Titanium fiber plates for bone tissue repair. Adv. Mater. 30, 1703608 (2018). - PubMed
-
- I. Yadroitsava, A. Du Plessis, I. Yadroitsev, Bone regeneration on implants of titanium alloys produced by laser powder bed fusion: A review, in Titanium for Consumer Applications (Elsevier, 2019), chap. 12, pp. 197–233.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

