Flares of acute graft-versus-host disease: a Mount Sinai Acute GVHD International Consortium analysis
- PMID: 38324721
- PMCID: PMC11103178
- DOI: 10.1182/bloodadvances.2023012091
Flares of acute graft-versus-host disease: a Mount Sinai Acute GVHD International Consortium analysis
Abstract
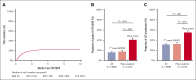
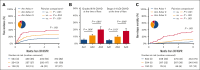
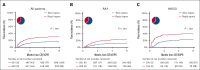
The absence of a standardized definition for graft-versus-host disease (GVHD) flares and data on its clinical course are significant concerns. We retrospectively evaluated 968 patients across 23 Mount Sinai Acute GVHD International Consortium (MAGIC) transplant centers who achieved complete response (CR) or very good partial response (VGPR) within 4 weeks of treatment. The cumulative incidence of flares within 6 months was 22%, and flares were associated with a higher risk of nonrelapse mortality (NRM; adjusted hazard ratio [aHR], 4.84; 95% confidence interval [CI], 3.19-7.36; P < .001). Flares were more severe (grades 3/4, 41% vs 16%; P < .001) and had more frequent lower gastrointestinal (LGI) involvement (55% vs 32%; P < .001) than the initial GVHD. At CR/VGPR, elevated MAGIC biomarkers predicted the future occurrence of a flare, along with its severity and LGI involvement. In multivariate analyses, higher Ann Arbor (AA) biomarker scores at CR/VGPR were significant risk factors for flares (AA2 vs AA1: aHR, 1.81 [95% CI, 1.32-2.48; P = .001]; AA3 vs AA1: aHR, 3.14 [95% CI, 1.98-4.98; P < .001]), as were early response to initial treatment (aHR, 1.84; 95% CI, 1.21-2.80; P = .004) and HLA-mismatched unrelated donor (aHR, 1.74; 95% CI, 1.00-3.02; P = .049). MAGIC biomarkers also stratified the risk of NRM both at CR/VGPR and at the time of flare. We conclude that GVHD flares are common and carry a significant mortality risk. The occurrence of future flares can be predicted by serum biomarkers that may serve to guide adjustment and discontinuation of immunosuppression.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.A.G. reports receiving research and/or clinical trial support from Novartis, Servier, Vertex, Cellectis, and Tmunity/Kite, and being a member of study steering committees, consulting, or scientific advisory boards for Novartis, Allogene, Adaptimmune, Juno/Bristol Myers Squibb, CRISPR/Vertex, Jazz, Kyttaro, and Cabaletta. M.W. received consulting fees from Amgen (Munich, Germany) and speaker’s fees from Novartis (Nürnberg, Germany). J.E.L. reports research support from Equillium, Incyte, MaaT Pharma, and Mesoblast, and consulting fees from bluebird bio, Editas, Equillium, Inhibrx, Kamada, Mesoblast, Sanofi, and X4 Pharmaceuticals. J.E.L. and J.L.M.F. are coinventors on a GVHD biomarker patent. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Steroid tapering after GVHD Rx: not too fast, not too slow.Blood Adv. 2024 Apr 23;8(8):2044-2046. doi: 10.1182/bloodadvances.2024012850. Blood Adv. 2024. PMID: 38652484 Free PMC article. No abstract available.
References
-
- Martin PJ. How I treat steroid-refractory acute graft-versus-host disease. Blood. 2020;135(19):1630–1638. - PubMed
-
- MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–394. - PubMed
-
- MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412–5417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

