Crenolanib and Intensive Chemotherapy in Adults With Newly Diagnosed FLT3-Mutated AML
- PMID: 38324741
- PMCID: PMC11107896
- DOI: 10.1200/JCO.23.01061
Crenolanib and Intensive Chemotherapy in Adults With Newly Diagnosed FLT3-Mutated AML
Abstract
Purpose: Crenolanib is a second-generation tyrosine kinase inhibitor with activity against FLT3-ITD- and TKD-mutant AML. We conducted a trial of crenolanib plus intensive chemotherapy in adults with newly diagnosed FLT3-mutant AML.
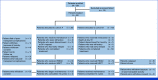
Methods: Eligible patients were 18 years and older. Induction chemotherapy consisted of cytarabine (100 mg/m2) continuous infusion on days 1-7 and anthracycline (daunorubicin 60-90 mg/m2 or idarubicin 12 mg/m2, once daily) on days 1-3 followed by consolidation with high-dose cytarabine (1-3 g/m2 twice daily on days 1, 3, 5) and/or allogeneic transplant. Crenolanib (100 mg thrice a day) was given from day 9 until 72 hours before the next cycle, after consolidation, and for 12 months after consolidation or transplant.
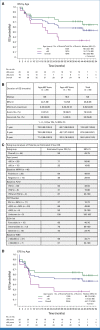
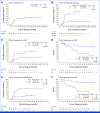
Results: Forty-four patients (median age, 57; range, 19-75 years) were enrolled. Thirty-six had FLT3-ITD, and 11 had FLT3-TKD mutations. European LeukemiaNet 2017 disease risk was favorable in 34%, intermediate in 30%, and adverse in 36%. The overall response rate was 86% (complete remission [CR], 77%; CR with incomplete count recovery [CRi], 9%): 90% in patients 60 years and younger and 80% in older patients. Measurable residual disease-negative CR/CRi rates were 89% and 45%, respectively. With a 45-month follow-up, median overall survival has not been reached and the median event-free survival was 44.7 months. Among younger patients, the estimated 3-year survival was 71.4% with 15% cumulative incidence of relapse. Treatment-related serious adverse events included febrile neutropenia, diarrhea, and nausea. The median time to platelets ≥100,000/µL and absolute neutrophil count ≥1,000/µL during induction was 29 and 32 days, respectively. No new FLT3-mutant clones were detected at relapse in patients completing consolidation.
Conclusion: Crenolanib plus intensive chemotherapy in adults with newly diagnosed FLT3-mutant AML results in high rate of deep responses and long-term survival with acceptable toxicity. A randomized trial of crenolanib versus midostaurin plus chemotherapy in younger patients is ongoing.
Trial registration: ClinicalTrials.gov NCT02283177.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Kottaridis P, Gale R, Frew M, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. - PubMed
-
- Fröhling S, Schlenk R, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: A study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. - PubMed
-
- Schlenk R, Kayser S, Bullinger L, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–3449. - PubMed
-
- Yalniz F, Abou Dalle I, Kantarjian H, et al. Prognostic significance of baseline FLT3-ITD mutant allele level in acute myeloid leukemia treated with intensive chemotherapy with/without sorafenib. Am J Hematol. 2019;94:984–991. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

