Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine
- PMID: 38324766
- PMCID: PMC11326847
- DOI: 10.1093/infdis/jiad593
Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine
Abstract
Background: MF59-adjuvanted gB subunit (gB/MF59) vaccine demonstrated approximately 50% efficacy against human cytomegalovirus (HCMV) acquisition in multiple clinical trials, suggesting that efforts to improve this vaccine design might yield a vaccine suitable for licensure.
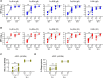
Methods: A messenger RNA (mRNA)-based vaccine candidate encoding HCMV gB and pentameric complex (PC), mRNA-1647, is currently in late-stage efficacy trials. However, its immunogenicity has not been compared to the partially effective gB/MF59 vaccine. We assessed neutralizing and Fc-mediated immunoglobulin G (IgG) effector antibody responses induced by mRNA-1647 in both HCMV-seropositive and -seronegative vaccinees from a first-in-human clinical trial through 1 year following third vaccination using a systems serology approach. Furthermore, we compared peak anti-gB antibody responses in seronegative mRNA-1647 vaccinees to that of seronegative gB/MF59 vaccine recipients.
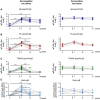
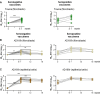
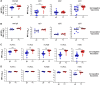
Results: mRNA-1647 vaccination elicited and boosted HCMV-specific IgG responses in seronegative and seropositive vaccinees, respectively, including neutralizing and Fc-mediated effector antibody responses. gB-specific IgG responses were lower than PC-specific IgG responses. gB-specific IgG and antibody-dependent cellular phagocytosis responses were lower than those elicited by gB/MF59. However, mRNA-1647 elicited higher neutralization and antibody-dependent cellular cytotoxicity (ADCC) responses.
Conclusions: Overall, mRNA-1647 vaccination induced polyfunctional and durable HCMV-specific antibody responses, with lower gB-specific IgG responses but higher neutralization and ADCC responses compared to the gB/MF59 vaccine.
Clinical trials registration: NCT03382405 (mRNA-1647) and NCT00133497 (gB/MF59).
Keywords: durability; gB/MF59 vaccine; human cytomegalovirus; mRNA-1647 vaccine; pentametric complex.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. S. R. P. is a consultant to Moderna, Merck, Pfizer, GSK, Dynavax, and Hoopika CMV vaccine programs and leads sponsored programs with Moderna and Merck. S. R. P. also serves on the board of the National CMV Foundation and as an educator on CMV for Medscape. E. B. W. has received funding support from Pfizer, Moderna, Sequiris, Najit Technologies, and Clinetic for the conduct of clinical trials and clinical research. E. B. W. has served as an advisor to Vaxcyte and consultant to ILiAD biotechnologies. J. H., M. K., K. W., L. P., and A. C. have Moderna company stocks. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Shenk TE, Stinski MF. Human cytomegalovirus. Preface. Curr Top Microbiol Immunol 2008; 325:v. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

