Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): multicentre randomised controlled trial
- PMID: 38325873
- PMCID: PMC11134408
- DOI: 10.1136/bmj-2023-076506
Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): multicentre randomised controlled trial
Erratum in
-
Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): multicentre randomised controlled trial.BMJ. 2024 May 1;385:q988. doi: 10.1136/bmj.q988. BMJ. 2024. PMID: 38692662 Free PMC article. No abstract available.
Abstract
Objective: To evaluate whether a structured online supervised group physical and mental health rehabilitation programme can improve health related quality of life compared with usual care in adults with post-covid-19 condition (long covid).
Design: Pragmatic, multicentre, parallel group, superiority randomised controlled trial.
Setting: England and Wales, with home based interventions delivered remotely online from a single trial hub.
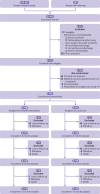
Participants: 585 adults (26-86 years) discharged from NHS hospitals at least three months previously after covid-19 and with ongoing physical and/or mental health sequelae (post-covid-19 condition), randomised (1:1.03) to receive the Rehabilitation Exercise and psycholoGical support After covid-19 InfectioN (REGAIN) intervention (n=298) or usual care (n=287).
Interventions: Best practice usual care was a single online session of advice and support with a trained practitioner. The REGAIN intervention was delivered online over eight weeks and consisted of weekly home based, live, supervised, group exercise and psychological support sessions.
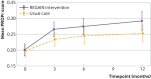
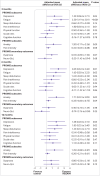
Main outcome measures: The primary outcome was health related quality of life using the patient reported outcomes measurement information system (PROMIS) preference (PROPr) score at three months. Secondary outcomes, measured at three, six, and 12 months, included PROMIS subscores (depression, fatigue, sleep disturbance, pain interference, physical function, social roles/activities, and cognitive function), severity of post-traumatic stress disorder, general health, and adverse events.
Results: Between January 2021 and July 2022, 39 697 people were invited to take part in the study and 725 were contacted and eligible. 585 participants were randomised. Mean age was 56 (standard deviation (SD) 12) years, 52% were female participants, mean health related quality of life PROMIS-PROPr score was 0.20 (SD 0.17), and mean time from hospital discharge was 323 (SD 144) days. Compared with usual care, the REGAIN intervention led to improvements in health related quality of life (adjusted mean difference in PROPr score 0.03 (95% confidence interval 0.01 to 0.05), P=0.02) at three months, driven predominantly by greater improvements in the PROMIS subscores for depression (1.39 (0.06 to 2.71), P=0.04), fatigue (2.50 (1.19 to 3.81), P<0.001), and pain interference (1.80 (0.50 to 3.11), P=0.01). Effects were sustained at 12 months (0.03 (0.01 to 0.06), P=0.02). Of 21 serious adverse events, only one was possibly related to the REGAIN intervention. In the intervention group, 141 (47%) participants fully adhered to the programme, 117 (39%) partially adhered, and 40 (13%) did not receive the intervention.
Conclusions: In adults with post-covid-19 condition, an online, home based, supervised, group physical and mental health rehabilitation programme was clinically effective at improving health related quality of life at three and 12 months compared with usual care.
Trial registration: ISRCTN registry ISRCTN11466448.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. GM, MU, JB, HS, KS, and JY are chief investigators or co-investigators on multiple previous and current research grants from the UK National Institute for Health and Care Research. GM and SE are directors of Atrium Health, a non-profit cardiopulmonary rehabilitation provider, which provided the treatment hub for the Rehabilitation Exercise and psycholoGical support After covid-19 InfectioN (REGAIN) trial. Both receive payment for their work as directors. HS and SP are directors of Health Psychology Services, a private health psychology provider. DM is a physiotherapist delivering post-intensive care unit rehabilitation for the NHS. MU is a current or recent co-investigator on Arthritis Research UK, Australian National Health and Medical Research Council, and Norwegian Medical Research Council grants; a director and shareholder of Clinvivo, which provides electronic data collection for health services research; part of an academic partnership with Serco, funded by the European Social Fund, related to return-to-work initiatives, and a co-investigator on two current and one recent National Institute for Health and Care Research (NIHR) funded studies receiving additional support from Stryker. JB has received travel expenses for speaking at conferences from the professional organisations hosting these conferences. JY is an existing member of the NIHR Health Technology Assessment General Funding Committee. KS was a member of the NIHR Health and Social Care Delivery Research Board from 2010 to 2018.
Figures
Comment in
-
Rehabilitation for post-covid-19 condition.BMJ. 2024 Feb 7;384:q20. doi: 10.1136/bmj.q20. BMJ. 2024. PMID: 38325886 No abstract available.
References
-
- Institute for Health Metrics and Evaluation. At least 17 million people in the WHO European Region experienced long COVID in the first two years of the pandemic; millions may have to live with it for years to come. https://www.healthdata.org/news-release/who-least-17-million-people-who-.... World Health Organization (WHO); 2022.
-
- Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 30 March 2023. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/...: ONS website, statistical bulletin; 2023.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
