Rna Buffering Fluorogenic Probe for Nucleolar Morphology Stable Imaging And Nucleolar Stress-Generating Agents Screening
- PMID: 38326089
- PMCID: PMC11022735
- DOI: 10.1002/advs.202309743
Rna Buffering Fluorogenic Probe for Nucleolar Morphology Stable Imaging And Nucleolar Stress-Generating Agents Screening
Abstract
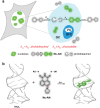
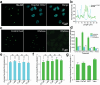
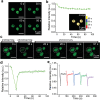
In the realm of cell research, membraneless organelles have become a subject of increasing interest. However, their ever-changing and amorphous morphological characteristics have long presented a formidable challenge when it comes to studying their structure and function. In this paper, a fluorescent probe Nu-AN is reported, which exhibits the remarkable capability to selectively bind to and visualize the nucleolus morphology, the largest membraneless organelle within the nucleus. Nu-AN demonstrates a significant enhancement in fluorescence upon its selective binding to nucleolar RNA, due to the inhibited twisted intramolecular charge-transfer (TICT) and reduced hydrogen bonding with water. What sets Nu-AN apart is its neutral charge and weak interaction with nucleolus RNA, enabling it to label the nucleolus selectively and reversibly. This not only reduces interference but also permits the replacement of photobleached probes with fresh ones outside the nucleolus, thereby preserving imaging photostability. By closely monitoring morphology-specific changes in the nucleolus with this buffering fluorogenic probe, screenings for agents are conducted that induce nucleolar stress within living cells.
Keywords: RNA. imaging; buffering; fluorogenic probes; nucleolus morphology.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- a) Caragine C. M., Haley S. C., Zidovska A., Phys. Rev. Lett. 2018, 121, 141801; - PMC - PubMed
- b) Caragine C. M., Haley S. C., Zidovska A., Elife 2019, 8, e47533; - PMC - PubMed
- c) Dubois M.‐L., Boisvert F.‐M., in The Functional Nucleus, (Eds: Bazett‐Jones D. P., Dellaire G.), Springer International Publishing, Cham, 2016, pp. 29–49.
-
- Boisvert F.‐M., van Koningsbruggen S., Navascués J., Lamond A. I., Nat. Rev. Mol. Cell Biol. 2007, 8, 574. - PubMed
-
- Corman A., Sirozh O., Lafarga V., Fernandez‐Capetillo O., Trends Biochem. Sci. 2023, 48, 274. - PubMed
-
- a) Boulon S., Westman B. J., Hutten S., Boisvert F.‐M., Lamond A. I., Mol. Cell 2010, 40, 216; - PMC - PubMed
- b) Mitrea D. M., Mittasch M., Gomes B. F., Klein I. A., Murcko M. A., Nat. Rev. Drug Discov. 2022, 21, 841; - PMC - PubMed
- c) Yang K., Yang J., Yi J., Cell Stress 2018, 2, 125. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
