Mitochondrial function in peripheral blood cells across the human lifespan
- PMID: 38326348
- PMCID: PMC10850142
- DOI: 10.1038/s41514-023-00130-4
Mitochondrial function in peripheral blood cells across the human lifespan
Abstract
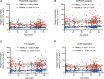
Mitochondrial dysfunction is considered a hallmark of aging. Up to now, a gradual decline of mitochondrial respiration with advancing age has mainly been demonstrated in human muscle tissue. A handful of studies have examined age-related mitochondrial dysfunction in human blood cells, and only with small sample sizes and mainly in platelets. In this study, we analyzed mitochondrial respiration in peripheral blood mononuclear cells (PBMCs) and platelets from 308 individuals across the human lifespan (0-86 years). In regression analyses, with adjustment for false discovery rate (FDR), we found age-related changes in respiratory measurements to be either small or absent. The main significant changes were an age-related relative decline in complex I-linked respiration and a corresponding rise of complex II-linked respiration in PBMCs. These results add to the understanding of mitochondrial dysfunction in aging and to its possible role in immune cell and platelet senescence.
© 2024. The Author(s).
Conflict of interest statement
J.E., E.Å.F., M.K., and E.E. have equity interests in and/or have received salary support from Abliva AB (formerly NeuroVive Pharmaceutical AB), a public company developing pharmaceuticals in the field of mitochondrial medicine. The remaining authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources

