Embryo-endometrial interaction associated with the location of the embryo during the mobility phase in mares
- PMID: 38326534
- PMCID: PMC10850102
- DOI: 10.1038/s41598-024-53578-z
Embryo-endometrial interaction associated with the location of the embryo during the mobility phase in mares
Abstract
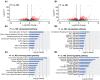
Embryo-maternal crosstalk is essential to establish pregnancy, with the equine embryo moving throughout the uterus on days 9-15 (ovulation = day 0) as part of this interaction. We hypothesized that the presence of a mobile embryo induces local changes in the gene expression of the endometrium. On Day 12, the endometrial transcripts were compared among three groups: uterine horn with an embryo (P+, n = 7), without an embryo (P-, n = 7) in pregnant mares, and both uterine horns of nonbred mares (NB, n = 6). We identified 1,101 differentially expressed genes (DEGs) between P+ vs. NB and 1,229 DEGs between P- vs. NB. The genes upregulated in both P+ and P- relative to NB were involved in growth factor pathway and fatty acid activation, while downregulated genes were associated with oxytocin signaling pathway and estrogen receptor signaling. Comparing the transcriptome of P+ to that of P-, we found 59 DEGs, of which 30 genes had a higher expression in P+. These genes are associated with regulating vascular growth factors and the immune system, all known to be essential in early pregnancy. Overall, this study suggests that the mobile embryo influences the endometrial gene expression locally.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Neely, D. P., Liu, I. K. & Hillman, R. B. Equine Reproduction. (Hoffmann-La Roche Inc., New Jersey., 1983).
-
- Hershman L, Douglas RH. The critical period for the maternal recognition of pregnancy in pony mares. J. Reprod. Fertil. Suppl. 1979;1:395–401. - PubMed

