A distinct cortical code for socially learned threat
- PMID: 38326610
- PMCID: PMC11856618
- DOI: 10.1038/s41586-023-07008-1
A distinct cortical code for socially learned threat
Abstract
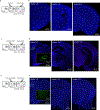
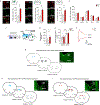
Animals can learn about sources of danger while minimizing their own risk by observing how others respond to threats. However, the distinct neural mechanisms by which threats are learned through social observation (known as observational fear learning1-4 (OFL)) to generate behavioural responses specific to such threats remain poorly understood. The dorsomedial prefrontal cortex (dmPFC) performs several key functions that may underlie OFL, including processing of social information and disambiguation of threat cues5-11. Here we show that dmPFC is recruited and required for OFL in mice. Using cellular-resolution microendoscopic calcium imaging, we demonstrate that dmPFC neurons code for observational fear and do so in a manner that is distinct from direct experience. We find that dmPFC neuronal activity predicts upcoming switches between freezing and moving state elicited by threat. By combining neuronal circuit mapping, calcium imaging, electrophysiological recordings and optogenetics, we show that dmPFC projections to the midbrain periaqueductal grey (PAG) constrain observer freezing, and that amygdalar and hippocampal inputs to dmPFC opposingly modulate observer freezing. Together our findings reveal that dmPFC neurons compute a distinct code for observational fear and coordinate long-range neural circuits to select behavioural responses.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures






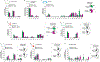





References
-
- Kondrakiewicz K, Kostecki M, Szadzinska W & Knapska E. Ecological validity of social interaction tests in rats and mice. Genes Brain Behav. 18, e12525 (2019). - PubMed
-
- Olsson A, Knapska E. & Lindstrom B. The neural and computational systems of social learning. Nat. Rev. Neurosci 21, 197–212 (2020). - PubMed
-
- Keum S. & Shin HS Rodent models for studying empathy. Neurobiol. Learn. Mem 135, 22–26 (2016). - PubMed
-
- Blanchard DC, Griebel G, Pobbe R. & Blanchard RJ Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev 35, 991–998 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

