Converting an allocentric goal into an egocentric steering signal
- PMID: 38326612
- PMCID: PMC10881393
- DOI: 10.1038/s41586-023-07006-3
Converting an allocentric goal into an egocentric steering signal
Abstract
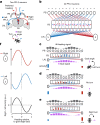
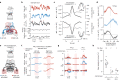
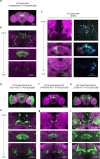
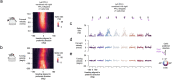
Neuronal signals that are relevant for spatial navigation have been described in many species1-10. However, a circuit-level understanding of how such signals interact to guide navigational behaviour is lacking. Here we characterize a neuronal circuit in the Drosophila central complex that compares internally generated estimates of the heading and goal angles of the fly-both of which are encoded in world-centred (allocentric) coordinates-to generate a body-centred (egocentric) steering signal. Past work has suggested that the activity of EPG neurons represents the fly's moment-to-moment angular orientation, or heading angle, during navigation2,11. An animal's moment-to-moment heading angle, however, is not always aligned with its goal angle-that is, the allocentric direction in which it wishes to progress forward. We describe FC2 cells12, a second set of neurons in the Drosophila brain with activity that correlates with the fly's goal angle. Focal optogenetic activation of FC2 neurons induces flies to orient along experimenter-defined directions as they walk forward. EPG and FC2 neurons connect monosynaptically to a third neuronal class, PFL3 cells12,13. We found that individual PFL3 cells show conjunctive, spike-rate tuning to both the heading angle and the goal angle during goal-directed navigation. Informed by the anatomy and physiology of these three cell classes, we develop a model that explains how this circuit compares allocentric heading and goal angles to build an egocentric steering signal in the PFL3 output terminals. Quantitative analyses and optogenetic manipulations of PFL3 activity support the model. Finally, using a new navigational memory task, we show that flies expressing disruptors of synaptic transmission in subsets of PFL3 cells have a reduced ability to orient along arbitrary goal directions, with an effect size in quantitative accordance with the prediction of our model. The biological circuit described here reveals how two population-level allocentric signals are compared in the brain to produce an egocentric output signal that is appropriate for motor control.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

