The HSP90-MYC-CDK9 network drives therapeutic resistance in mantle cell lymphoma
- PMID: 38326887
- PMCID: PMC10848414
- DOI: 10.1186/s40164-024-00484-9
The HSP90-MYC-CDK9 network drives therapeutic resistance in mantle cell lymphoma
Abstract
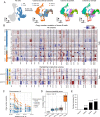
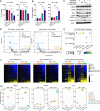
Brexucabtagene autoleucel CAR-T therapy is highly efficacious in overcoming resistance to Bruton's tyrosine kinase inhibitors (BTKi) in mantle cell lymphoma. However, many patients relapse post CAR-T therapy with dismal outcomes. To dissect the underlying mechanisms of sequential resistance to BTKi and CAR-T therapy, we performed single-cell RNA sequencing analysis for 66 samples from 25 patients treated with BTKi and/or CAR-T therapy and conducted in-depth bioinformatics™ analysis. Our analysis revealed that MYC activity progressively increased with sequential resistance. HSP90AB1 (Heat shock protein 90 alpha family class B member 1), a MYC target, was identified as early driver of CAR-T resistance. CDK9 (Cyclin-dependent kinase 9), another MYC target, was significantly upregulated in Dual-R samples. Both HSP90AB1 and CDK9 expression were correlated with MYC activity levels. Pharmaceutical co-targeting of HSP90 and CDK9 synergistically diminished MYC activity, leading to potent anti-MCL activity. Collectively, our study revealed that HSP90-MYC-CDK9 network is the primary driving force of therapeutic resistance.
Keywords: CAR-T therapy; Mantle cell lymphoma; Resistance; Single-cell RNA sequencing.
© 2024. The Author(s).
Conflict of interest statement
MW is consultant to AstraZeneca, BeiGene, BioInvent, CSTone, Deciphera, DTRM Biopharma (Cayman) Limited, Epizyme, Genentech, InnoCare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Miltenyi Biomedicine GmbH, Oncternal, Pepromene Bio, Pharmacyclics, VelosBio. MW has received research support from Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genmab, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, VelosBio, Vincerx. MW received a speaker honoraria from Acerta Pharma, Anticancer Association, AstraZeneca, BeiGene, BGICS, BioInvent, CAHON, Clinical Care Options, Dava Oncology, Eastern Virginia Medical School, Epizyme, Hebei Cancer Prevention Federation, Imedex, Janssen, Kite Pharma, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, Miltenyi Biomedicine GmbH, Moffit Cancer Center, Mumbai Hematology Group, OMI, OncLive, Pharmacyclics, Physicians Education Resources (PER), Practice Point Communications (PPC), The First Afflicted Hospital of Zhejiang University. ZZ is a consultant of Melax.
Figures





References
-
- Song YQ, Zhou KS, Zou DH, Zhou JF, Hu JD, Yang HY, et al. Safety and activity of the investigational Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with mantle cell lymphoma from a phase 2 trial. Blood. 2018;132(Suppl 1):148. doi: 10.1182/blood-2018-99-117956. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

