Injectable ultrasound-powered bone-adhesive nanocomposite hydrogel for electrically accelerated irregular bone defect healing
- PMID: 38326903
- PMCID: PMC10851493
- DOI: 10.1186/s12951-024-02320-y
Injectable ultrasound-powered bone-adhesive nanocomposite hydrogel for electrically accelerated irregular bone defect healing
Abstract
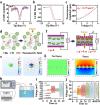
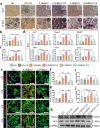
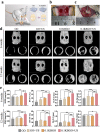
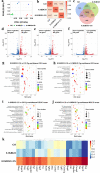
The treatment of critical-size bone defects with irregular shapes remains a major challenge in the field of orthopedics. Bone implants with adaptability to complex morphological bone defects, bone-adhesive properties, and potent osteogenic capacity are necessary. Here, a shape-adaptive, highly bone-adhesive, and ultrasound-powered injectable nanocomposite hydrogel is developed via dynamic covalent crosslinking of amine-modified piezoelectric nanoparticles and biopolymer hydrogel networks for electrically accelerated bone healing. Depending on the inorganic-organic interaction between the amino-modified piezoelectric nanoparticles and the bio-adhesive hydrogel network, the bone adhesive strength of the prepared hydrogel exhibited an approximately 3-fold increase. In response to ultrasound radiation, the nanocomposite hydrogel could generate a controllable electrical output (-41.16 to 61.82 mV) to enhance the osteogenic effect in vitro and in vivo significantly. Rat critical-size calvarial defect repair validates accelerated bone healing. In addition, bioinformatics analysis reveals that the ultrasound-responsive nanocomposite hydrogel enhanced the osteogenic differentiation of bone mesenchymal stem cells by increasing calcium ion influx and up-regulating the PI3K/AKT and MEK/ERK signaling pathways. Overall, the present work reveals a novel wireless ultrasound-powered bone-adhesive nanocomposite hydrogel that broadens the therapeutic horizons for irregular bone defects.
Keywords: Bone adhesive; Bone defects; Electrical stimulation; Injectability and self-healing; Nanocomposite hydrogel.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Huang WJ, Cheng S, Wang XL, Zhang Y, Chen LY, Zhang LN. Noncompressible Hemostasis and Bone Regeneration Induced by an Absorbable Bioadhesive Self-Healing Hydrogel. Adv Funct Mater. 2021;31:2009189. doi: 10.1002/adfm.202009189. - DOI
MeSH terms
Substances
Grants and funding
- 82202662/National Natural Science Foundation of China
- 2023A1515010784/Basic and Applied Basic Research Foundation of Guangdong Province
- 2020B1515120062/Basic and Applied Basic Research Foundation of Guangdong Province
- 2021A0505030083/Guangdong Provincial Science and Technology Program
- U22A20316/Regional Innovation and Development Joint Fund of National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

