High-dose systemic adeno-associated virus vector administration causes liver and sinusoidal endothelial cell injury
- PMID: 38327046
- PMCID: PMC11163197
- DOI: 10.1016/j.ymthe.2024.02.002
High-dose systemic adeno-associated virus vector administration causes liver and sinusoidal endothelial cell injury
Abstract
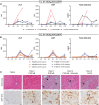
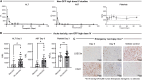
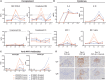
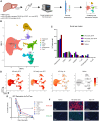
We analyzed retrospective data from toxicology studies involving administration of high doses of adeno-associated virus expressing different therapeutic transgenes to 21 cynomolgus and 15 rhesus macaques. We also conducted prospective studies to investigate acute toxicity following high-dose systemic administration of enhanced green fluorescent protein-expressing adeno-associated virus to 10 rhesus macaques. Toxicity was characterized by transaminitis, thrombocytopenia, and alternative complement pathway activation that peaked on post-administration day 3. Although most animals recovered, some developed ascites, generalized edema, hyperbilirubinemia, and/or coagulopathy that prompted unscheduled euthanasia. Study endpoint livers from animals that recovered and from unscheduled necropsies of those that succumbed to toxicity were analyzed via hypothesis-driven histopathology and unbiased single-nucleus RNA sequencing. All liver cell types expressed high transgene transcript levels at early unscheduled timepoints that subsequently decreased. Thrombocytopenia coincided with sinusoidal platelet microthrombi and sinusoidal endothelial injury identified via immunohistology and single-nucleus RNA sequencing. Acute toxicity, sinusoidal injury, and liver platelet sequestration were similarly observed with therapeutic transgenes and enhanced green fluorescent protein at doses ≥1 × 1014 GC/kg, suggesting it was the consequence of high-dose systemic adeno-associated virus administration, not green fluorescent protein toxicity. These findings highlight a potential toxic effect of high-dose intravenous adeno-associated virus on nonhuman primate liver microvasculature.
Keywords: AAV; NHP; TMA; adeno-associated virus; endothelium; liver; nonhuman primate; thrombotic microangiopathy; toxicity.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.M.W. is a paid advisor to and holds equity in iECURE, Scout Bio, Passage Bio, and the Center for Breakthrough Medicines (CBM). He also holds equity in the former G2 Bio asset companies. He has sponsored research agreements with Amicus Therapeutics, CBM, Elaaj Bio, FA212, Foundation for Angelman Syndrome Therapeutics, former G2 Bio asset companies, iECURE, Passage Bio, and Scout Bio, which are licensees of Penn technology. J.H., J.A.G., and J.M.W. are inventors on patents that have been licensed to various biopharmaceutical companies and for which they may receive payments.
Figures


References
-
- Chand D.H., Zaidman C., Arya K., Millner R., Farrar M.A., Mackie F.E., Goedeker N.L., Dharnidharka V.R., Dandamudi R., Reyna S.P. Thrombotic Microangiopathy Following Onasemnogene Abeparvovec for Spinal Muscular Atrophy: A Case Series. J. Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. - DOI - PubMed
-
- Chand D.H., Mitchell S., Sun R., LaMarca N., Reyna S.P., Sutter T. Safety of Onasemnogene Abeparvovec for Spinal Muscular Atrophy Patients Heavier than 8.5 kg in a Global Managed Access Program. Pediatr. Neurol. 2022;132:27–32. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

