Neoadjuvant chemotherapy for breast cancer: Pathologic response rates but not tumor size, has an independent prognostic impact on survival
- PMID: 38327130
- PMCID: PMC10904968
- DOI: 10.1002/cam4.6930
Neoadjuvant chemotherapy for breast cancer: Pathologic response rates but not tumor size, has an independent prognostic impact on survival
Abstract
Aim: We investigated the pathologic complete response rates (pCR) and survival outcomes of early breast cancer patients who underwent neoadjuvant chemotherapy (NAC) over 14 years at a French comprehensive cancer center and reported pCR and survival outcomes by tumor subtypes and size.
Methods: From January 2005 to December 2018, 1150 patients receiving NAC were identified. Correlations between cT stage, breast tumor response, axillary lymph node response, pCR, surgery, and outcomes were assessed. pCR was defined as (ypT0/ypTis) and (ypN0/pN0sn).
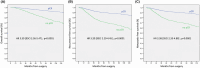
Results: A pCR was reached in 31.7% (365/1150) of patients and was strongly associated with tumor subtypes, but not with tumor size (pretreatment cT category). Luminal-B Her2-negative and triple-negative (TN) subtypes, cN1 status, older age, and no-pCR had an independent negative prognostic value. Overall survival (OS), relapse-free survival (RFS), and metastasis-free survival (MFS) were not significantly different for cT0-1 compared to cT2 stages. In Cox-model adjusted on in-breast pCR and pN status, ypN1 had a strong negative impact (OS, RFS, and MFS: HR = 3.153, 4.677, and 6.133, respectively), higher than no in-breast pCR (HR = 2.369, 2.252, and 2.323). A negative impact of no pCR on OS was observed for cN0 patients and TN tumors (HR = 4.972) or HER2-positive tumors (HR = 11.706), as well as in Luminal-B Her2-negative tumors on MFS (HR = 2.223) and for Luminal-A on RFS (HR = 4.465) and MFS (HR = 4.185).
Conclusion: Achievement of pCR, but not tumor size (pretreatment cT category), has an independent prognostic impact on survival. These results suggest potential NAC benefits in patients with small tumors (<2 cm), even in absence of clinically suspicious lymph nodes. Residual lymph node disease after NAC is the most powerful adverse prognostic factor.
Trial registration: ClinicalTrials.gov NCT02869607.
Keywords: breast cancer; neoadjuvant chemotherapy; pathologic response; survival.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Alexandre de Nonneville declares Consulting fees by Gilead, Seagen, Lilly, and Novartis, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events by Gilead, Daiichi Sankyo, and MSD, Support for attending meetings and/or travel by Gilead, Lilly, and Daiichi Sankyo. Emmanuelle Charaffe declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events by Exact Science (to institution), Veracyte (to institution), and AstraZeneca. Anthony Gonçalves declares Support for attending meetings and/or travel by Menarini, Participation on a Data Safety Monitoring Board or Advisory Board by Novartis, MSD, and Daiichi Sankyo. No conflict of interest was declared by others authors.
Figures

References
-
- Taucher S, Steger GG, Jakesz R, et al. The potential risk of neoadjuvant chemotherapy in breast cancer patients—results from a prospective randomized trial of the Austrian breast and colorectal cancer study group (ABCSG‐07). Breast Cancer Res Treat. 2008;112(2):309‐316. - PubMed
-
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine‐year results from national surgical adjuvant breast and bowel project B‐18. J Natl Cancer Inst Monogr. 2001;30:96‐102. - PubMed
-
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana‐Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224‐4237. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

