Exploring Promising Therapies for Non-Alcoholic Fatty Liver Disease: A ClinicalTrials.gov Analysis
- PMID: 38327733
- PMCID: PMC10847589
- DOI: 10.2147/DMSO.S448476
Exploring Promising Therapies for Non-Alcoholic Fatty Liver Disease: A ClinicalTrials.gov Analysis
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is a common disease and has been increasing in recent years. To date, no FDA-approved drug specifically targets NAFLD.
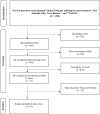
Methods: The terms "Non-alcoholic Fatty Liver Disease" and "NAFLD" were used in a search of ClinicalTrials.gov on August 24, 2023. Two evaluators independently examined the trials using predetermined eligibility criteria. Studies had to be interventional, NAFLD focused, in Phase IV, and completed to be eligible for this review.
Results: The ClinicalTrials.gov database was searched for trials examining pharmacotherapeutics in NAFLD. The search revealed 1364 trials, with 31 meeting the inclusion criteria. Out of these, 19 were finalized for evaluation. The dominant intervention model was Parallel. The most prevalent studies were in Korea (26.3%) and China (21.1%). The most common intervention was metformin (12.1%), with others like Exenatide and Pioglitazone accounting for 9.1%.
Conclusion: Therapeutics used to manage NAFLD are limited. However, various medications offer potential benefits. Further investigations are definitely warranted.
Keywords: NAFLD; clinical trials; hepatology; metabolic disorder; therapeutics.
© 2024 Hegazi et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Shahwan M, Jairoun AA, Alaila RFF, et al. Relation of erythrocyte sedimentation rate, glycemic parameters and lipid profile for the prediction of major adverse cardiovascular events: a single-center, cross-sectional study in Palestine. Obes Med. 2023;43:100513. doi:10.1016/j.obmed.2023.100513 - DOI
-
- Role of insulin resistance in nonalcoholic fatty liver disease | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic. Available from: https://academic.oup.com/jcem/article/91/12/4753/2656230. Accessed August 24, 2023.
Publication types
LinkOut - more resources
Full Text Sources