Therapeutic Role of Synthetic Lethality in ARID1A-Deficient Malignancies
- PMID: 38327752
- PMCID: PMC10846636
- DOI: 10.36401/JIPO-22-37
Therapeutic Role of Synthetic Lethality in ARID1A-Deficient Malignancies
Abstract
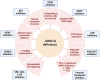
AT-rich interaction domain 1A (ARID1A), a mammalian switch/sucrose nonfermenting complex subunit, modulates several cellular processes by regulating chromatin accessibility. It is encoded by ARID1A, an immunosuppressive gene frequently disrupted in a many tumors, affecting the proliferation, migration, and invasion of cancer cells. Targeting molecular pathways and epigenetic regulation associated with ARID1A loss, such as inhibiting the PI3K/AKT pathway or modulating Wnt/β-catenin signaling, may help suppress tumor growth and progression. Developing epigenetic drugs like histone deacetylase or DNA methyltransferase inhibitors could restore normal chromatin structure and function in cells with ARID1A loss. As ARID1A deficiency correlates with enhanced tumor mutability, microsatellite instability, high tumor mutation burden, increased programmed death-ligand 1 expression, and T-lymphocyte infiltration, ARID1A-deficient cells can be a potential therapeutic target for immune checkpoint inhibitors that warrants further exploration. In this review, we discuss the role of ARID1A in carcinogenesis, its crosstalk with other signaling pathways, and strategies to make ARID1A-deficient cells a potential therapeutic target for patients with cancer.
Keywords: ARID1A; EZH2; PARP; PIK3CA; synthetic lethality.
Figures
References
-
- Versteege I,, Sévenet N,, Lange J,, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer Nature 1998. 394 203–206 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
