Limited Independent Follow-Up with Germline Testing of Variants Detected in BRCA1 and BRCA2 by Tumor-Only Sequencing
- PMID: 38327755
- PMCID: PMC10846638
- DOI: 10.36401/JIPO-23-2
Limited Independent Follow-Up with Germline Testing of Variants Detected in BRCA1 and BRCA2 by Tumor-Only Sequencing
Abstract
Introduction: Genomic profiling is performed in patients with advanced or metastatic cancer, in order to direct cancer treatment, often sequencing tumor-only, without a matched germline comparator. However, because many of the genes analyzed on tumor profiling overlap with those known to be associated with hereditary cancer predisposition syndromes (HCPS), tumor-only profiling can unknowingly uncover germline pathogenic (P) and likely pathogenic variants (LPV). In this study, we evaluated the number of patients with P/LPVs identified in BRCA1 and BRCA2 (BRCA1/2) via tumor-only profiling, then determined the germline testing outcomes for those patients.
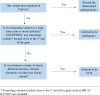
Methods: A retrospective chart review was performed to identify patients with BRCA1/2 variants on tumor-only genomic profiling, and whether they had germline testing.
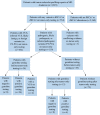
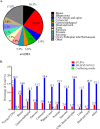
Results: This study found that of 2923 patients with 36 tumor types who underwent tumor-only testing, 554 had a variant in BRCA1/2 (19.0%); 119 of the 554 patients (21.5%) had a P/LP BRCA1/2 variant, representing 4.1% of the overall population who underwent genomic profiling. Seventy-three (61.3%) of 119 patients with BRCA1/2 P/LPV on tumor-only testing did not undergo germline testing, 34 (28.6%) had already had germline testing before tumor-only testing, and 12 (10.1%) underwent germline testing after tumor-only testing. Twenty-eight germline BRCA1/2 P/LPVs were detected, 24 in those who had prior germline testing, and 4 among the 12 patients who had germline testing after tumor-only testing.
Conclusion: Tumor-only testing is likely to identify P/LPVs in BRCA1/2. Efforts to improve follow-up germline testing is needed to improve identification of germline BRCA1/2 alterations.
Keywords: BRCA1; BRCA2; germline testing; tumor-only testing.
Conflict of interest statement
Conflict of Interest: The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships may not relate to the subject matter of this manuscript. Ecaterina E. Ileana Dumbrava reports the following: Consulting: Catamaran Bio; Advisory Committee: BOLT Therapeutics; and Sponsored Research (to the institution): Bayer HealthCare Pharmaceuticals Inc., Immunocore LTD, Amgen, Aileron Therapeutics, Compugen LTD, TRACON Pharmaceuticals Inc., Unum Therapeutics, Immunomedics, BOLT Therapeutics, Aprea Therapeutics, Bellicum Pharmaceuticals, PMV Pharma, Triumvira, Seagen Inc, Mereo BioPharma 5 Inc., Sanofi, and Astex Therapetics. Ying Yuan reports the following: Consulting: AbbVie, Amgen, Bexion Pharmaceuticals, BeyondSpring Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol Myers Squibb, GT Medical Technologies, Mirati Therapeutics, Servier Pharmaceuticals, Starpax Pharmaceuticals, and Vertex Pharmaceuticals; and Honoraria: Blueprint. Milind Javle reports the following: Advisory Committee: OrgiMed, More Health, EDO; Sponsored Research (self): QED, Novartis, and Meclun; Sponsored Research (to the institution): Lily and Arqule; Honoraria: Taiho, Seattle Genetics, Merck. Jordi Rodon Ahnert reports the following: Advisory Committee: Novartis, Eli Lilly, Orion Pharmaceuticals, Servier Pharmaceuticals, Peptomyc, Merck Sharp & Dohme, Kelun Pharmaceuticals/Klus Pharma, Spectrum Pharmaceuticals, Inc., Pfizer, Roche Pharmaceuticals, Ellipses Pharma, Certera, Bayer, Molecular Partners, NovellusDX, IONCTURA SA, Kisoji Biotechnology, Inc.; Sponsored Research (to the institution): Bayer, Novartis, Blueprint Medicines, Spectrum Pharmaceuticals, Tocagen, Symphogen, BioAlta, Pfizer, GenMab, CytomX, Kelun-Biotech, Takeda-Millenniums, GlaxoSmithKline, Ipsen; Travel Reimbursement: ESMO, Department of Defense, Merck Sharp & Dohme, Louisiana State University, Kelun Pharmaceuticals/Klus Pharma, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, Bayer, WIN Consortium, Janssen, Molecular Partners; Other: European Journal of Cancer, VHIO/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, SOLTI, Elsevier, GlaxoSmithKline. Timothy A. Yap reports the following: Consulting: AstraZeneca, Almac, Aduro, Artios, Athena, Atrin, Axiom, Bayer, Bristol Myers Squibb, Calithera, Clovis, Cybrexa, EMD Serono, F-Star, GLG, Guidepoint, Ignyta, I-Mab, ImmuneSensor, Jansen, Merck, Pfizer, Repare, Roche, Schrodinger, Seattle Genetics, Varian, Zai Labs, and ZielBio; Sponsored Research (to the institution): AstraZeneca, Artios, Bayer, Beigene, BioNTech, BMS, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, Haihe, ImmuneSensor, Ionis, Ipsen, Jounce, Karyopharm, KSQ, Kyowa, Merck, Novartis, Pfizer, Repare, Ribon Therapeutics, Regeneron, Rubius, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, and Vivace. Banu Arun reports the following: Sponsored Research (to the institution): AstraZeneca; and Advisory Committee: AbbVie (non-paid steering committee member for BROCADE 3 study). Funda Meric-Bernstam reports the following: Consulting: AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, Daiichi Sankyo Co. Ltd., DebioPharm, Ecor1 Capital, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., GT Apeiron, Genentech Inc., Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, Menarini Group, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Protai Bio Ltd, Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks; Advisory Committee: Black Diamond, Biovica, Eisai, FogPharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, Zentalis; Sponsored Research (to the institution): Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co.; Honoraria: Chugai Biopharmaceuticals; and Other (Travel Related): European Organisation for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO). The remaining authors have no disclosures.
Figures
References
-
- Li MM,, Datto M,, Duncavage EJ,, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists J Mol Diagn 2017. 19 4–23 - PMC - PubMed
-
- Petrucelli N,, Daly MB,, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2 Genet Med 2010. 12 245–259 - PubMed
-
- Cobain EF,, Jacobs M,, Wu Y-M,, et al. Tumor/normal genomic profiling in patients with metastatic solid tumors identifies pathogenic germline variants of therapeutic importance J Clin Oncol 2020. 38 (15_suppl): 1501 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
