Selective recognition and extraction of iodide from pure water by a tripodal selenoimidazol(ium)-based chalcogen bonding receptor
- PMID: 38327780
- PMCID: PMC10847689
- DOI: 10.1016/j.isci.2024.108917
Selective recognition and extraction of iodide from pure water by a tripodal selenoimidazol(ium)-based chalcogen bonding receptor
Abstract
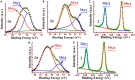
A selenium-based tripodal chalcogen bond (ChB) donor TPI-3Se is demonstrated for the recognition and extraction of I- from 100% water medium. NMR and ITC studies with the halides reveal that the ChB donor selectively binds with the large, weakly hydrated I-. Interestingly, I- crystallizes out selectively in the presence of other halides supporting the superiority of the selective recognition of I-. The X-ray structure of the ChB-iodide complex manifests both the μ1 and μ2 coordinated interactions, which is rare in the C-Se···I chalcogen bonding. Furthermore, to validate the selective I- binding potency of TPI-3Se in pure water, comparisons are made with its hydrogen and halogen bond donor analogs. The computational analysis also provides the mode of I- recognition by TPI-3Se. Importantly, this receptor is capable of extracting I- from pure water through selenium sigma-hole and I- interaction with a high degree of efficiency (∼70%).
Keywords: Chemistry; Inorganic chemistry; Natural sciences.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Busschaert N., Caltagirone C., Van Rossom W., Gale P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015;115:8038–8155. - PubMed
-
- Chen L., Berry S.N., Wu X., Howe E.N., Gale P.A. Advances in anion receptor chemistry. Chem. 2020;6:61–141.
-
- Erdélyi M. Halogen bonding in solution. Chem. Soc. Rev. 2012;41:3547–3557. - PubMed
-
- Gale P., Howe E., Wu X. Anion receptor chemistry. Chem. 2016;1:351–422.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

