A single cell atlas of circulating immune cells involved in diabetic retinopathy
- PMID: 38327792
- PMCID: PMC10847734
- DOI: 10.1016/j.isci.2024.109003
A single cell atlas of circulating immune cells involved in diabetic retinopathy
Abstract
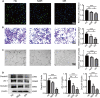
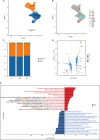
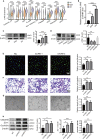
This study focused on examining the exact role of circulating immune cells in the development of diabetic retinopathy (DR). In vitro co-culture experiments showed that peripheral blood mononuclear cells (PBMCs) from patients with type 1 DR crucially modulated the biological functions of human retinal microvascular endothelial cells (HRMECs), consequently disrupting their normal functionality. Single-cell RNA sequencing (scRNA-seq) study revealed unique differentially expressed genes and pathways in circulating immune cells among healthy controls, non-diabetic retinopathy (NDR) patients, and DR patients. Of significance was the observed upregulation of JUND in each subset of PBMCs in patients with type 1 DR. Further studies showed that downregulating JUND in DR patient-derived PBMCs led to the amelioration of HRMEC dysfunction. These findings highlighted the notable alterations in the transcriptomic patterns of circulating immune cells in type 1 DR patients and underscored the significance of JUND as a key factor for PBMCs in participating in the pathogenesis of DR.
Keywords: Cell biology; Components of the immune system; Gene network; Transcriptomics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Teo Z.L., Tham Y.C., Yu M., Chee M.L., Rim T.H., Cheung N., Bikbov M.M., Wang Y.X., Tang Y., Lu Y., et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580–1591. doi: 10.1016/j.ophtha.2021.04.027. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

