Trial staff and community member perceptions of barriers and solutions to improving racial and ethnic diversity in clinical trial participation; a mixed method study
- PMID: 38328002
- PMCID: PMC10847850
- DOI: 10.1016/j.conctc.2024.101262
Trial staff and community member perceptions of barriers and solutions to improving racial and ethnic diversity in clinical trial participation; a mixed method study
Abstract
Background: The lack of racial and ethnic diversity in clinical trials leads to skewed findings, limited generalizability, inequitable health outcomes for people of color, and insufficient access to innovative therapies. Our objective was to compare perceptions of barriers to participation in trials for people of color and trial staff to provide tangible solutions for improving diversity among study participants.
Methods: This mixed method study utilized semi-structured interviews and surveys to evaluate barriers to participation and solutions to improve racial and ethnic diversity in clinical trials among healthcare system trial staff and community members from the same region. Through thematic analysis via coded transcripts and quantitative analysis via survey data, social support theory constructs were identified to evaluate where perceptions of barriers and solutions overlap and where they diverge.
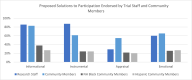
Results: A total of 55 trial staff and 75 community members participated in the study. Trial staff identified logistics and patients' unwillingness to receive additional treatments as perceived barriers to participation, while community members stated lack of information and lack of trust in their care team. Both groups identified hesitance toward research as a prominent barrier. Solutions related to informational support demonstrated the most overlap between groups, while instrumental support showed the most discordance.
Conclusion: Solutions for improving racial and ethnic diversity in clinical trial participation are multi-faceted and have various levels of impact. Overlap and discordance of opinions regarding solutions should be further evaluated, and implementation of solutions should be carefully considered.
Keywords: Barriers to participation; Clinical trials participation; Diversity in clinical trials; Mixed method; Solutions for participation.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Improving diversity in study participation: Patient perspectives on barriers, racial differences and the role of communities.Health Expect. 2022 Aug;25(4):1979-1987. doi: 10.1111/hex.13554. Epub 2022 Jun 28. Health Expect. 2022. PMID: 35765232 Free PMC article.
-
Involving South Asian patients in clinical trials.Health Technol Assess. 2004 Oct;8(42):iii, 1-109. doi: 10.3310/hta8420. Health Technol Assess. 2004. PMID: 15488164 Review.
-
Barriers to diverse clinical trial participation in Duchenne muscular dystrophy: Engaging Hispanic/Latina caregivers and health professionals.Orphanet J Rare Dis. 2024 May 21;19(1):207. doi: 10.1186/s13023-024-03209-7. Orphanet J Rare Dis. 2024. PMID: 38773664 Free PMC article.
-
Community engagement and clinical trial diversity: Navigating barriers and co-designing solutions-A report from the "Health Equity through Diversity" seminar series.PLoS One. 2023 Feb 16;18(2):e0281940. doi: 10.1371/journal.pone.0281940. eCollection 2023. PLoS One. 2023. PMID: 36795792 Free PMC article.
-
Improving Racial and Ethnic Equity in Clinical Trials Enrolling Pregnant and Lactating Individuals.J Clin Pharmacol. 2023 Jun;63 Suppl 1:S21-S33. doi: 10.1002/jcph.2263. J Clin Pharmacol. 2023. PMID: 37317498 Review.
Cited by
-
Patient participation in clinical trials conducted by principal investigators who speak one or more language(s) beyond english: Exploring ethnicity as proxy for language.Contemp Clin Trials Commun. 2024 Aug 17;41:101353. doi: 10.1016/j.conctc.2024.101353. eCollection 2024 Oct. Contemp Clin Trials Commun. 2024. PMID: 39252860 Free PMC article.
-
Racial, ethnic, and socioeconomic disparities in clinical trial reporting for metastatic spine tumors: An exploration of North American studies.Neurosurg Rev. 2025 Feb 19;48(1):247. doi: 10.1007/s10143-025-03343-1. Neurosurg Rev. 2025. PMID: 39969615 Free PMC article. Review.
-
Fact or Myth? Black Patients Do Not Want to Participate in Clinical Trials.Clin Transl Gastroenterol. 2025 Apr 1;16(4):e00826. doi: 10.14309/ctg.0000000000000826. Clin Transl Gastroenterol. 2025. PMID: 39878425 Free PMC article.
-
The feasibility of the Community health worker Outreach and Navigation Network for Enhancing representation in Clinical Trials (CONNECT) model: A community-physician engagement approach for increasing representation in clinical trials.Contemp Clin Trials Commun. 2025 Jul 7;46:101521. doi: 10.1016/j.conctc.2025.101521. eCollection 2025 Aug. Contemp Clin Trials Commun. 2025. PMID: 40698153 Free PMC article.
-
A Formative Evaluation of Interventions to Enhance Clinical Trial Diversity Guided by the Socioecological Model.Cancers (Basel). 2025 Jul 9;17(14):2282. doi: 10.3390/cancers17142282. Cancers (Basel). 2025. PMID: 40723166 Free PMC article.
References
-
- Hayunga E.G., Pinn V.W. NIH policy on the inclusion of women and minorities as subjects in clinical research. Princ Pract Clin Res. 2002:145–160. doi: 10.1016/b978-012274065-7/50013-7. - DOI
-
- FDA . 2020. Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry.
-
- FDA . 2022. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials.
LinkOut - more resources
Full Text Sources