This is a preprint.
Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates
- PMID: 38328053
- PMCID: PMC10849624
- DOI: 10.1101/2024.01.23.576837
Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates
Update in
-
Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates.Cell. 2025 Jul 24;188(15):4123-4140.e18. doi: 10.1016/j.cell.2025.04.039. Epub 2025 May 23. Cell. 2025. PMID: 40412392
Abstract
Cytosolic aggregation of the nuclear protein TDP-43 is associated with many neurodegenerative diseases, but the triggers for TDP-43 aggregation are still debated. Here, we demonstrate that TDP-43 aggregation requires a double event. One is up-concentration in stress granules beyond a threshold, and the other is oxidative stress. These two events collectively induce intra-condensate demixing, giving rise to a dynamic TDP-43 enriched phase within stress granules, which subsequently transitions into pathological aggregates. Mechanistically, intra-condensate demixing is triggered by local unfolding of the RRM1 domain for intermolecular disulfide bond formation and by increased hydrophobic patch interactions in the C-terminal domain. By engineering TDP-43 variants resistant to intra-condensate demixing, we successfully eliminate pathological TDP-43 aggregates in cells. We conclude that up-concentration inside condensates and simultaneous exposure to environmental stress could be a general pathway for protein aggregation, with intra-condensate demixing constituting a key intermediate step.
Keywords: ALS; TDP-43; intra-condensate demixing; multiphasic condensate; neurodegenerative diseases; phase separation; protein aggregation; stress granules.
Figures


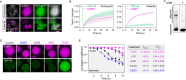



References
-
- Cosacak M.I., Bhattarai P., Bocova L., Dzewas T., Mashkaryan V., Papadimitriou C., Brandt K., Hollak H., Antos C.L., and Kizil C. (2017). Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain. Sci. Rep. 7, 12959. 10.1038/s41598-017-13311-5. - DOI - PMC - PubMed
-
- Guo J.L., Buist A., Soares A., Callaerts K., Calafate S., Stevenaert F., Daniels J.P., Zoll B.E., Crowe A., Brunden K.R., et al. (2016). The Dynamics and Turnover of Tau Aggregates in Cultured Cells: INSIGHTS INTO THERAPIES FOR TAUOPATHIES. J. Biol. Chem. 291, 13175–13193. 10.1074/jbc.M115.712083. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
